Norkolut: how to take the drug
Method of administration and dose.
The effectiveness of Norkolut tablets may be reduced if the patient forgets to take the tablet as directed. The patient should take only the last missed tablet as soon as she remembers, and then continue taking the tablets at the usual time the next day.
Secondary amenorrhea
The doctor prescribes a drug containing estrogens (for example, for 14 days) before Norkolut is started. After this, take 1 tablet of Norkolut 1-2 times a day for 10 days. Withdrawal bleeding begins a few days after taking the last pill.
When a sufficient level of estrogen production is achieved, you can try to stop estrogen therapy and induce cyclic bleeding using 1 Norkolut tablet 2 times a day from the 16th to the 25th day of the cycle.
Endometriosis
Treatment should begin between the 1st and 5th day of the cycle using 1 tablet of Norkolut twice a day. If bleeding occurs, it is necessary to increase the dose and take 2 tablets of Norkolut twice a day. After bleeding has stopped, you can reduce the dose to the initial dose. The duration of treatment is at least 4-6 months. With continuous daily use of the drug, ovulation and menstruation are usually absent. After completion of hormonal therapy, withdrawal bleeding occurs.
Features of application.
To prevent pregnancy, it is necessary to use non-hormonal methods of contraception.
It is necessary to consult a doctor as soon as possible in the following cases:
- any changes in health status, especially those indicated in these instructions;
- chest compressions;
- the need to use other medications;
- prolonged immobilization or the need for surgery (in case of planned surgery at least 6 weeks before surgery);
- unusually excessive vaginal bleeding.
https://youtu.be/Be-tnjVmlnU
Contraindications
The drug should not be used for adolescents, in the presence of malignant tumors and acute allergic reactions. It is prescribed with caution if there are problems with the liver, kidneys, diabetes, frequent migraine attacks, elevated blood sugar, epileptic seizures, asthma, and increased blood clotting.
When prescribing Norkolut, compatibility with other medications is taken into account. It is not recommended to use it with long-acting barbiturates, hormonal drugs of the steroid group, or drugs that thicken the blood.
With proper calculation of dosages and duration of use, Norkolut has an excellent therapeutic effect, especially with menopausal syndrome in women over 40 years of age.
Norkolut - contraindications, side effects
Contraindications.
Norkolut should not be used if you have any of the conditions or diseases listed below.
- Pregnancy or suspicion of it.
- Lactation.
- Formation of a blood clot in a vein (thrombosis), such as in the blood vessels of the legs (deep vein thrombosis) or lungs (pulmonary embolism).
- High risk of venous or arterial thrombosis.
- Conditions that precede thrombosis (including transient ischemic attack, angina) currently or in history.
- History of migraine with focal neurological symptoms.
- Diseases associated with poor circulation in the arteries, such as myocardial infarction or stroke, now or in the past.
- Diabetes mellitus with vascular complications.
- Current or past severe liver disease until liver function tests return to normal. Symptoms of liver disease may include, for example, yellowing of the skin and/or itching of the entire body.
- Dubin-Johnson syndrome, Rotor syndrome, and jaundice or cases of severe itching during previous pregnancies.
- Previous cases of pemphigoid in pregnancy (herpes of pregnancy).
- Benign or malignant liver tumors currently or in the past.
- Malignant tumors are dependent on the influence of sex hormones (for example, breast or genital organs), which are currently present or have been in the past.
- Vaginal bleeding of unknown etiology.
- Untreated endometrial hyperplasia.
- Hypersensitivity to norethisterone or to any of the auxiliary components of the drug.
If any of the above conditions occur for the first time while taking the drug, you should immediately stop taking it and consult your doctor.
Adverse reactions.
Side effects most often occur in the first months after starting to take Norkolut.
Tumors
Isolated cases of benign liver tumors have been described, and even more rarely, cases of malignant tumors in patients who take hormonal substances included in Norkolut. In some cases, these tumors led to life-threatening intra-abdominal bleeding.
Instructions for use NORCOLUT®
Before starting treatment with norethisterone, it is necessary to conduct a medical examination, including measurement of blood pressure, assessment of the condition of the mammary glands, abdominal and pelvic organs, as well as cytological examination of the cervix.
Norethisterone therapy should be discontinued immediately in the following cases:
- the development of migraine-type headaches or an increase in unusually severe headaches;
- sudden disturbances of perception (for example, visual or hearing disturbances);
- the first signs of thrombophlebitis or symptoms of thromboembolism, a feeling of pain and tightness in the chest;
- 6 weeks before the planned surgical intervention, as well as in case of prolonged immobilization;
- the occurrence of jaundice or deterioration of liver function, the development of an anicteric form of hepatitis, skin itching;
- significant increase in blood pressure;
- pregnancy.
If any of the following risk factors are present or occur during use of Norkolut®, an individual assessment of the risk/benefit ratio should be carried out before starting treatment or continuing to take the drug.
Vascular disorders
Based on the results of epidemiological studies, it was found that the use of oral estrogens/gestagens containing ovulation inhibitors is associated with an increase in the number of thromboembolic complications. Thus, one should keep in mind the possibility of their development, especially if there is a history of thromboembolic diseases.
If the patient experiences symptoms indicating the development of thromboembolic complications, you should immediately stop taking Norkolut®. The need for treatment should be reconsidered before continuing to use Norkolut®.
Well-established risk factors for venous thromboembolism (VTE) include:
- episodes of VTE or a family history (VTE in a brother, sister or parent at a relatively young age);
- age;
- obesity;
- SLE;
- long-term immobilization;
- extensive surgery;
- severe injuries.
Patients with a history of VTE or thrombophilic disorders are at increased risk of developing VTE. Treatment with Norkolut® may increase this risk.
A personal or family history of episodes of thromboembolism or recurrent spontaneous abortion should be carefully examined to exclude a predisposition to thromboembolism.
Unless a thorough assessment of thrombophilic factors has been performed or anticoagulant treatment has been initiated, the use of progestins in such patients is contraindicated. In patients already taking anticoagulants, the risks and benefits of progestin therapy should be carefully weighed.
The risk of VTE may temporarily increase with prolonged immobilization, severe trauma, or major surgery. In all patients after major surgery, special attention should be paid to prophylactic measures to prevent VTE. If prolonged immobilization is expected during elective surgery, especially in abdominal or orthopedic surgery of the lower extremities, it is necessary to consider suspending progestin therapy 4-6 weeks before surgery. Treatment should not be resumed until the patient has fully recovered motor activity.
Hyperlipidemia
Women with hypertriglyceridemia or a family history of hypertriglyceridemia may be at increased risk of developing pancreatitis when taking combined oral contraceptives (COCs).
Women with hypertriglyceridemia have an increased risk of developing arterial lesions. However, there is no need for routine screening of all women taking COCs.
Neoplasms
In rare cases, benign tumors, and even more rarely, malignant liver tumors have been reported in individuals taking hormonal drugs similar to Norkolut.
In some cases, these neoplasms have led to life-threatening intra-abdominal bleeding. If there are complaints of pain in the upper abdomen, liver enlargement, or signs of intra-abdominal bleeding, a liver tumor should be excluded and the drug should be discontinued.
Other Cautions
Norethisterone may affect carbohydrate metabolism. The parameters of carbohydrate metabolism must be carefully monitored in all patients with diabetes mellitus before and during treatment.
Chloasma can sometimes develop, especially in women with a history of chloasma during pregnancy. Women prone to chloasma should minimize sun exposure and exposure to ultraviolet radiation during treatment with norethisterone.
Patients with a history of episodes of depression should be closely monitored. Treatment with Norkolut® should be discontinued if severe depression recurs.
In any case of acute visual impairment, exophthalmos, diplopia or migraine, papilledema or retinal damage should be excluded before continuing treatment with Norkolut®.
Progestins can cause fluid retention. When prescribing Norkolut®, special attention should be paid to patients with the following diseases:
- epilepsy, migraine, asthma, heart disease, renal dysfunction.
Additional measures related to the partial metabolism of norethisterone to ethinyl estradiol
Following oral administration, norethisterone is partially metabolized to ethinyl estradiol at a dose equivalent to approximately 4-6 mcg ethinide estradiol per 1 mg oral norethisterone or norethisterone acetate. Due to the partial conversion of norethisterone to ethinyl estradiol, the use of Norkolut is expected to lead to similar pharmacological effects that occur with COCs. Therefore, the following should be taken into account:
- Arterial and venous thromboembolism
- severe pain in the calf muscle of one leg, swelling of the lower leg;
- sudden shortness of breath, chest pain.
- sudden severe chest pain, radiating or not radiating to the left arm;
- sudden cough for no apparent reason;
- any unusually strong, prolonged headache, especially when occurring for the first time. The condition gradually worsens or any of the following symptoms occur: sudden partial or complete loss of vision or double vision; aphasia; dizziness; collapse with or without attacks of focal epilepsy; weakness or severe sudden numbness of one side or one part of the body.
Epidemiological studies have shown that the incidence of venous thromboembolism (VTE) in women taking low-estrogen oral contraceptives (<50 mcg ethinyl estradiol) is approximately 20-40 per 100,000 cases per year, but this risk estimate varies depending on the content gestagen. By comparison, in women who do not take oral contraceptives, the incidence is 5-10 per 100,000 cases per year. The use of any COC carries an increased risk of VTE compared to patients who do not use them. This risk is less than the risk of pregnancy-associated VTE, which is estimated to occur in 60 cases per 100,000 pregnancies. The highest risk of VTE exists during the first year of COC use or after a break in use for a minimum period of 1 month. VTE can be life-threatening or fatal (in 1-2% of cases).
VTE in the form of deep vein thrombosis and/or pulmonary embolism can occur during the use of any COC. It is known that extremely rarely, when using COCs, thrombosis can occur in other blood vessels, for example, in the hepatic, mesenteric, renal, cerebral, as well as in the veins and arteries of the retina. There is no consensus as to whether the development of these complications is associated with the use of COCs.
Common symptoms of VTE include:
The use of COCs may also increase the risk of diseases such as stroke and myocardial infarction, which are secondary arterial thromboembolic complications.
Common symptoms of arterial thromboembolism include:
Risk factors for the development of thromboembolic complications:
- age;
- obesity (BMI >30 kg/m2);
- indication in the family history of the presence of venous or arterial thromboembolism in a brother, sister or parent at a relatively young age (if a hereditary predisposition is known or suspected, the woman should seek specialist advice before deciding to use COCs);
- prolonged immobilization, major surgery, any surgery on the lower extremities or major trauma (in these situations it is advisable to stop using the COC, in the case of elective surgery at least 4 weeks before it, and not to resume earlier than 2 weeks after full recovery);
- smoking (the risk increases with smoking and with age, especially in women over 35 years of age);
- dyslipoproteinemia;
- arterial hypertension;
- migraine (an increase in the frequency and severity of migraine attacks while taking COCs may be a harbinger of cerebrovascular accidents and, therefore, a reason for immediate cessation of taking COCs);
- heart valve defects;
- atrial fibrillation.
Other factors that cause circulatory disorders (diseases in which blood circulation is impaired):
- diabetes mellitus, SLE, hemolytic-uremic syndrome, chronic inflammatory bowel disease (Crohn's disease/ulcerative colitis), sickle cell anemia.
Biochemical factors that may indicate a hereditary or acquired predisposition to venous or arterial thrombosis:
- activated protein C resistance (APC), hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant).
Cervical cancer
The most important risk factor for developing cervical cancer is persistent HPV infection. Some epidemiological studies have suggested that long-term use of COCs may further contribute to increased risk, but there is still uncertainty due to the presence of additional factors (eg, sexual behavior, including use of barrier contraceptives).
Mammary cancer
A meta-analysis of 54 epidemiological studies found that there is a slightly increased relative risk (OP=1.24) of breast cancer in women who currently use COCs.
The increased risk gradually disappears within 10 years after stopping COC use. Because breast cancer is rare in women under 40 years of age, the increased incidence of breast cancer in current and past COC users is small relative to the risk of breast cancer in the general population. These studies do not provide evidence of cause and effect. The observed pattern of increased risk of breast cancer may be due to earlier cancer diagnosis in women taking COCs, biological effects of COCs, or a combination of both. In people who have ever taken COCs, breast cancer is diagnosed at earlier stages than in those patients who have never taken COCs.
Other factors
Although small increases in blood pressure have been reported in many women taking COCs, clinically significant increases are rare. However, if persistent clinically significant arterial hypertension develops during the use of a COC, it is advisable to discontinue the COC and begin antihypertensive therapy. If necessary, taking COCs can be resumed if antihypertensive therapy allows you to achieve target blood pressure values.
There are reports that Crohn's disease and ulcerative colitis may be associated with COC use.
Norkolut® contains lactose monohydrate. Patients with congenital galactose intolerance, lapp lactase deficiency or glucose-galactose malabsorption should not use the drug.
Impact on the ability to drive vehicles and operate machinery
Norethisterone does not affect the ability to drive vehicles or operate machinery.
Side effects
Before starting to use Norkolut, the patient must be examined for the presence of malignant neoplasms and have the mammary glands diagnosed.
Among the side effects of the tablets are:
- headache;
- nausea, vomiting;
- thrombosis - with long-term use;
- bleeding from the genital tract in the middle of the menstrual cycle;
- allergic reactions in the form of skin rashes;
- weight gain;
- breast tension;
- chronic fatigue.
To avoid the development of side effects, Norkolut should not be taken simultaneously with liver enzyme inducers, barbiturates, Rifampicin and other drugs that cause oxidation processes in the liver.
Prescribe with caution when treating with glucocorticosteroids, hypoglycemic drugs and oral anticoagulants. https://youtu.be/7qZpYCtv1Fs
Find out if pills help against uterine endometriosis.
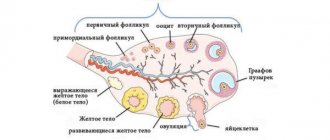
Effect of the drug Norkolut
As already mentioned, the active substance of Norkolut is norethisterone. This substance belongs to gestagens, but acts as androgens and estrogens. Of course, these properties are expressed to a lesser extent than in gestagens, which are produced by the body itself.
After detecting a hormonal imbalance (which often happens during pregnancy or menopause), the doctor immediately prescribes Norkolut for you. And the treatment begins.
After taking the pills, the medicine immediately enters the stomach and begins to break down. Thanks to starch and cellulose, Norkolut is instantly absorbed by the walls of the stomach and enters the blood, thereby normalizing the level of estrogen and androgens in the body.
The body immediately senses this. Menstruation is more gentle, pregnant women experience no pain in the lower abdomen, and menopause no longer brings constant mood swings. But that’s not all.
special instructions
It is not advisable to use hypoglycemic drugs, glucocorticosteroids, oral anticoagulants, as well as Cimetidine, Rifampicin and other drugs that affect microsomal liver enzymes with Norkolut.
Before starting therapy, the patient should undergo a complete and thorough examination of the mammary glands, as well as oncological and gynecological examinations to exclude possible oncological lesions.
If you accidentally miss another pill while taking Norkolut, the patient should take the missed dose of the drug as quickly as possible and use additional adequate methods of contraception.
Indications for use
According to the instructions for Norkolut, it is prescribed for treatment:
- Uterine fibroids;
- Mastodynia (pain in the mammary glands);
- Endometriosis;
- Endometrial hyperplasia (with cystic glandular changes);
- Premenstrual syndrome;
- Dysmenorrhea occurring with a decrease in the secretion phase;
- Anovulatory metrorrhagia.
The drug is also used when carrying out a progesterone test, contraception, to prevent or stop lactation.