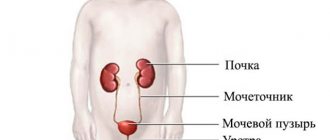
A blood test for ferritin is performed to determine iron stores. Of course, the iron we need so much is not present in the human body in its pure form, since even free iron atoms can be poisoned, since they are toxic.
And it is ferritin that we owe to the fact that the iron reserves in our body are presented in a non-toxic soluble form. The solubility of iron in the presence of ferritin increases enormously (100 trillion times). These features allowed ferritin to take one of the dominant roles in iron metabolism.
The diagnostic capabilities of ferritin are also known, allowing one to accurately identify iron deficiency conditions in the body.
What is ferritin
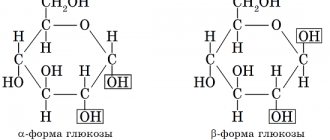
Specific protein F functions as a kind of storage of free iron, which is a toxic form for the body. It is a complex protein structure that is highly soluble in water. The molecule of this compound consists of a hollow shell, called apoferritin, and a crystalline core containing phosphate and hydroxide F.
Iron in ferritin is in a non-toxic, water-soluble and physiologically readily available state and makes up 1/5 of the entire molecule. This is approximately equal to 3,000-4,000 iron atoms. The shell includes 24 subunits, which are divided into 2 types - L (light - light) and H (heavy - heavy). They differ in molecular weight, synthesis characteristics and other characteristics.
The number of these components is not the same for different organs; for example, in the spleen and liver, the ferritin molecule predominantly consists of the L subunit (80-90%), while the H subunit occupies only 10-20%. But in the heart, cancerous tumors, placenta and fetal tissues (fetal tissues), most of the ferritin molecule is represented by the H subunit.
Reference! The reasons that determine the organ specificity of ferritin subunits are not fully understood, but many scientists are inclined to hypothesize that this feature is interconnected with their function. For example, hepatic ferritin is the main depot for the body, placental ferritin ensures the transfer of iron to the fetus from the mother, etc.
The production of this protein is carried out by the cells of the liver, bone marrow, spleen, thyroid gland, small intestine, kidneys, placenta with the participation of white blood cells - leukocytes. At the same time, it ensures the performance of the corresponding tissues.
Synthesized ferritin enters the blood serum and is found there in small quantities. Based on its origin, this compound is divided into 2 types - plasma ferritin, which enters the blood during the breakdown of serum cells, and tissue ferritin, which is released into the blood during the destruction of tissues in the cells of which ferritin is present.
Reasons for low ferritin
Having considered the symptoms of a condition in which ferritin is low and what this means, it becomes clear that the deviation significantly worsens a person’s well-being and reduces performance. If there is a lack of iron in the blood necessary to carry out vital processes, hemoglobin partially loses its ability to provide cellular structures with oxygen. A decrease in ferritin levels can occur for the following reasons:
- iron deficiency anemia - a disorder of hemoglobin synthesis caused by a lack of iron;
- prolonged diarrhea;
- gluten enteropathy (celiac disease) – a disorder of the digestive process as a result of damage to the villi of the small intestine;
- lack of iron in food;
- gastric bypass is a surgical intervention most often aimed at reducing body weight;
- heavy blood loss, including during menstruation;
- ulcerative formations of the duodenum or stomach;
- pregnancy;
- diseases of the endocrine system with dysfunction of the thyroid gland;
- multiple pregnancy;
- oncological lesions of various localizations;
- parasitic infections;
- intravascular hemolysis - premature breakdown of red blood cells;
- some diseases of the genital organs in women (uterine fibroids, endometriosis and others).
Deviations from the norm of ferritin can also occur for reasons that are not pathological disorders. For example, those at risk of reducing the concentration of iron in the body include donors and people with a deficiency of vitamin C. Dietary and vegetarian diets, addiction to bad habits, and consumption of large quantities of coffee and dairy products also lead to a decrease in the content of the microelement.
Main functions
Ferritin under physiological conditions is a kind of indicator of F content in the blood. It is known that 1 µg/l F in the blood corresponds to 8 milligrams of deposited iron. The primary function of this protein is the storage and accumulation of iron reserves, and its main depot is hepatic ferritin.
This protein belongs to the acute phase proteins, the reaction of which (pre-immune response) is formed to protect against pathogens, neutralizing or stopping the rate of their reproduction. When a microorganism is introduced, a full-fledged reaction develops over 5-7 days, so a pre-immune response is necessary to ensure the body’s protection until the main reaction of the immune system is formed.
For normal functioning, microorganisms also need iron. It is necessary for the production of enzymes (catalysts of chemical reactions). That is why, when a pathogen enters the blood serum, the F content is reduced, and this limits the possibility of microorganisms accessing it. Also, ions of this microelement can negatively affect one’s own immune cells.
For effective binding of serum iron, ferritin production increases and its content increases. During the preimmune response, the H-subunit takes the main part, since its ability to seize iron is higher than that of the stable L-subunit. It is also the first to protect cells from damage. Normally, the L content is higher than H, while in the acute phase response the opposite is true.
Reference! With any inflammatory process, no matter non-infectious or infectious, ferritin will always be elevated. However, its level very rarely exceeds 1000 µg/l. It is worth noting that often iron deficiency anemia can be hidden under inflammatory processes and can only be detected in a biochemical analysis.
The relationship between ferritin and hemoglobin
In some cases, low ferritin is found in the blood with normal hemoglobin. This condition may occur in the following situations:
- with iron deficiency anemia;
- for celiac disease;
- as a result of prolonged diarrhea;
- due to post-traumatic bleeding;
- for endocrine pathologies;
- after a woman gives birth;
- for ulcers and gastritis.
Therefore, hemoglobin level is not an indicator of normal iron content. For diagnosis, the amount of ferritin must be examined.
How to check the content of a substance in the blood
To find out the level of concentration of this protein in the body, just go to one of the laboratories and donate venous blood. As a rule, a referral for this test is prescribed by a hematologist, oncologist, hepatologist or local physician.
What is included in preparing for the test?
Quite a number of factors can affect iron levels and, therefore, ferritin. Therefore, before the procedure, you must consult with your doctor and follow all his recommendations regarding preparation for the examination. These include the following:
- 12 hours before blood sampling, you should refrain from eating, since blood must be donated on an empty stomach;
- half an hour before the examination, exclude emotional and physical stress;
- stop taking iron-containing medications a week before;
- stop smoking the day before the procedure.
In turn, the doctor who interprets the test results must be aware of multiple non-pathological factors that can lead to changes in iron and ferritin levels.
Is it possible to undergo examination during menstruation?
During menstruation, donating blood for iron and ferritin, like most other tests, is not recommended - this can lead to distorted results. During menstruation, there is often a decrease in hemoglobin concentration and an increase in the content of red blood cells. Before and during menstruation, the level of serum F increases, and at the end it returns to normal levels. Therefore, it is better to refrain from taking the test during this period, and undergo the test about a week after the end of your period.
Interesting facts about ferritin
Ferritin is elevated
In contrast to low ferritin, there are many more reasons for an increased level of iron-containing protein:
- Overload of the body with iron, including as a result of uncontrolled use of appropriate medications (hemosiderosis, hemochromatosis).
- Blood transfusions, especially repeated ones over a short period of time.
- Acute and chronic liver diseases (viral and drug-induced hepatitis, cirrhosis of various etiologies, necrosis, alcoholic lesions, obstructive jaundice), neoplastic process in the liver parenchyma (hepatoma, cancer metastases from other organs).
- Diseases of the blood system (polycythemia, anemia of various origins: hemolytic, sideroblastic, aplastic, pernicious or caused by another chronic process).
- Oncological processes affecting the blood system (myelo- and lymphoblastic leukemia, lymphogranulomatosis) and other organs (breast cancer), metastatic damage to the bone marrow.
- Collagenosis: rheumatoid arthritis, SLE (systemic lupus erythematosus).
- Cardiovascular pathology associated with circulatory disorders (myocardial infarction, stroke).
- Elevated ferritin is observed in the case of acute inflammatory diseases (as an “acute phase protein”): infections of the respiratory system, bones, urinary tract, as well as burns and febrile conditions.
Diagnostic capabilities
Studying the level of ferritin in the blood makes it possible to obtain a detailed picture of the state of iron metabolic processes. Decoding the results allows you to:
What does MCV mean in a blood test?
- assessment of iron reserves - normally and in various pathologies (renal failure, bleeding, hemodialysis);
- differential diagnosis of anemia (decreased hemoglobin level) - to distinguish iron deficiency anemia from chronic diseases in which hemoglobin is reduced;
- determination of latent (hidden) F deficiency, that is, detect deviations before the onset of characteristic symptoms and a decline in hemoglobin concentration;
- control of therapy with iron preparations - on the 3-5th day of taking medications, the ferritin level should increase by 50 mg/dl or more, otherwise it is necessary to diagnose the presence of bleeding.
In most situations, a blood test for ferritin is prescribed during periodic examinations, as well as in complex diagnostics to identify any disease during the patient’s initial treatment. As a rule, in the second case, the patient has symptoms of pathologies arising from impaired iron metabolism, such as:
- increased fatigue, constant feeling of tiredness, irritability;
- pale skin, brittle and split nails, hair loss;
- decreased immunity, tachycardia (rapid heartbeat);
- chronic bleeding (nose or gums), heavy menstruation;
- muscle pain in the absence of excessive physical activity;
- dysfunction of the digestive organs - heartburn, nausea, vomiting;
- swelling and pain in the joints;
- increased skin pigmentation;
- decreased sex drive.
With such symptoms and complaints, the subjects often show deviations in ferritin levels in one direction or another, which confirms the doctor’s suspicions about the presence of the disease.
Ferritin level test
To determine the test, blood from a vein is required
The content of this protein in blood plasma is determined using biochemical analysis. For the study, blood is collected from a vein.
A ferritin test can be prescribed by doctors in different situations:
- decreased hemoglobin or red blood cell count;
- any signs of iron deficiency (systematic dizziness, general weakness of the body, nausea, shaking limbs, fainting, etc.);
- any signs of iron deficiency (abdominal pain, joint pain, body aches, heart rhythm disturbances);
- control of iron levels in case of concomitant diseases;
- pregnancy;
- donation
Normal ferritin levels
The level of ferritin in the blood varies depending on the gender and age characteristics of patients, and to a greater extent on gender characteristics. In newborns, this figure is quite high, and during the first two months there is an even increase. Whereas in a later period, infants experience a decrease in its concentration.
For about a year, the values that determine the concentration of this protein increase and remain at approximately the same level until adulthood. In men, the rate is significantly higher than in women from puberty until full maturity. Studies have shown that the highest concentration of F is observed in males aged 30-39 years.
Women are characterized by a lower level, and until the onset of menopause it will be lower. Then the ferritin concentration gradually increases and stops approximately at the same limits as those of an adult man. This change is due to the physiological characteristics of the female body.
Ferritin norms for different categories of patients
During monthly blood loss, pregnancy, and childbirth, a woman needs an increased amount of iron, which is used almost immediately and is not stored in reserve. And since ferritin shows deposited iron, its parameters will accordingly be smaller. The content of ferritin in blood serum is not affected by bad habits (for example, smoking), as well as the place of residence of a person.
Ferritin norms in children and adults
The recognized normal level of ferritin, which determines the concentration of iron in the blood, varies according to age.
Comparison of age and normative ferritin level:
- up to 1 month: 25-205 mcg/l;
- 1 month - year: 100-600 mcg/l;
- 1-10 years: 55-90 µg/l;
- 10-13 years: 30-55 µg/l;
- 13-17 years: 35-155 µg/l.
A healthy adult body synthesizes ferritin differently: more depending on the biological sex of the person than on age:
- for women the norm is 16-110 mcg/l,
- for men - 25-310 mcg/l.
When reaching old age, the normal amount of ferritin levels out again for representatives of either sex. They correspond to 25-240 µg/l.
What is ferritin level during pregnancy?
Iron is necessary for the full development of the fetus during pregnancy.
During pregnancy, a woman actively uses iron reserves by the body, so ferritin levels are greatly reduced. To monitor indicators, it is advisable to take blood tests several times at different stages of pregnancy:
- in the first trimester of pregnancy, the norm is 50-90 mcg/l;
- in the second trimester - 30-50 mcg/l;
- immediately before birth, the rate drops to 12-16 μg/l.
Too low values of ferritin indicators must be monitored with the help of doctors in order to prevent the influence of its deficiency on the development of the fetus, as well as childbirth and the course of the postpartum period for the woman.
Iron consumption during multiple pregnancy is increased
Reasons why ferritin levels may be extremely low during pregnancy:
- Unbalanced diet.
- Multiple pregnancy.
- Fourth birth (or more).
- The previous births were less than two years ago.
- Persistent menstrual irregularities before pregnancy.
- Bleeding.
- Chronic diseases of the gastrointestinal tract.
- Oncological diseases.
If the ferritin level of a pregnant woman greatly exceeds the norm, this also urgently needs to be taken under control. The reasons may be:
- Hepatitis.
- Various pathological blood conditions.
- Disturbances in iron metabolism in the body.
- Acute leukemia.
- Autoimmune diseases.
- Oncological diseases.
- Acute infectious lesions in the body.
- Pathologies of the cardiovascular system.
- Blood transfusion.
Why might the rate decrease?
Reduced ferritin in the blood is observed in a fairly wide range of pathological conditions and diseases. If there is a lack of iron due to an unbalanced diet, blood loss or diseases of the digestive system, the body uses iron reserves from this protein, which causes a decrease in its concentration. Thus, the following pathologies can reduce the F level.
Hypothyroidism
Thyroid dysfunction leads to decreased stomach acidity. This prevents the transformation of 3-valent iron into 2-valent iron, which is available for absorption by the body. Hypothyroidism also negatively affects the absorption of folic acid and vitamin B12, which prevents their absorption in the gastrointestinal tract (gastrointestinal tract). Considering that iron is necessary for the normal synthesis of thyroid hormones, the result is a vicious circle.
Increased need for iron
The body's increased need for F is often combined with a decrease in ferritin concentration. Thus, rapid growth of the body, pregnancy, uncontrolled donation, and excessive physical and mental stress can reduce its level.
Blood loss
With various types of bleeding, iron is lost from hemoglobin. Such a deficiency can be caused by chronic (from the gums or nose) or regular (heavy menstruation) blood loss.
Gastrointestinal diseases
Atrophic gastritis, enterocolitis, celiac disease and other pathologies, accompanied by inflammation of the mucous membrane and damage to the villi of the tract, prevent the absorption of iron and its transformation.
Hypovitaminosis and unbalanced nutrition
Iron is not produced in the body, so you can get it only from food or from a specially designed medicine. With a monotonous and vitamin-poor diet, hypovitaminosis develops, which reduces the body’s ability to absorb F. As a result, its own reserves are used, and the concentration of deposited ferritin drops.
Menopause
During menopause, many metabolic processes, including those related to iron metabolism, fade away.
Pregnancy (3rd trimester) and breastfeeding
During this period, the female body requires more iron, and if its consumption is not sufficiently replenished, then the reserves from the depot are depleted.
The main signs of low iron and ferritin in the blood
Prevention measures
- A nutritious, varied diet. Eating foods containing iron (liver, red meat, rabbit, turkey, eggs, mussels, mackerel, seaweed, oysters, apples, pears, pomegranates, apricots, beans, peas, almonds, mushrooms, buckwheat and oatmeal, dark chocolate and etc.), as well as B vitamins (nuts, seafood, lean meat, milk, kefir, cheese, cottage cheese, etc.) and vitamin C (citrus fruits, bell peppers, black currants, rose hips, etc.).
- Undergo periodic and regular medical examinations at your place of residence or place of work in order to notice deviations in time and take action.
- If the signs described above appear, be sure to consult a doctor promptly. It is better to treat the disease at the beginning than in the advanced phase.
- Lead a healthy lifestyle - spend more time in the fresh air, play sports, do not drink alcohol and drugs, do not eat fast food and synthetic foods. Move more, drink clean water in sufficient quantities, follow a sleep schedule.
For the proper functioning of the organs and systems of the body, a regular and sufficient supply of oxygen is required. The delivery of these important compounds to cells is carried out by red blood cells. The vital activity and functioning of blood cells responsible for supplying oxygen depends on the level of iron. The storage element in the body is protein – ferritin. It is present in all cellular structures, but is more concentrated in the spleen, liver and blood as part of red blood cells. If ferritin is low, what this means is not clear to every patient. We will consider the causes, symptoms and methods of treatment for deviations in the level of the indicator in more detail.
What is a ferritin blood test?
When examining ferritin, what a blood test means, a doctor can decipher it, and this indicator plays an important diagnostic value in identifying many pathologies.
Ferritin analysis is often prescribed for the purpose of differential diagnosis of iron deficiency anemia and other types of anemia that develop in a number of chronic pathologies.
MORE ABOUT: Diet during pregnancy for weight loss - menu
Determining the level of this compound is a direct analysis that makes it possible to distinguish an absolute deficiency of ferrum from a relative one, which may arise due to a violation of the transition of the element from the depot.
To understand why patients are prescribed this test, we list what ferritin in the blood shows:
- quantitative level of iron deficiency;
- hidden (latent) ferrum deficiency;
- excess iron in the body;
- dynamics of growth of malignant tumors and monitoring of their treatment;
- determination of the type of anemic condition;
- assessment of the effectiveness of treatment with iron-containing medications.
Due to the fact that the level of ferritin in the blood depends on many external factors, before taking blood for analysis, a number of rules should be followed to avoid distortion of the results as much as possible. Preparation for analysis includes:
- discontinuation of iron-containing medications two weeks before the study date;
- avoiding drinking alcohol or smoking cigarettes the day before the test;
- avoiding intense physical activity and stressful situations half an hour before blood sampling;
- avoiding the consumption of coffee and strong tea 12 hours before the study;
- abolition of food 12 hours before the procedure (only drinking non-carbonated purified water is allowed);
- rescheduling the analysis in case of menstruation in women (it is optimal to take blood samples a week after the end of the critical days).
It is worth considering that a false increase or decrease in ferritin can be caused by taking the following medications (if you are taking them, you must inform the doctor prescribing the study):
- oral contraceptives;
- antitumor drugs (Asparaginase, Methotrexate);
- medications with estrogen or testosterone;
- antibiotics (Cefotaxime, Chloramphenicol);
- iron-containing medications (Hematogen, Sorbifer, Totema);
- non-steroidal anti-inflammatory drugs (Aspirin);
- lipid-lowering drugs (Cholestyramine, Metformin);
- glucocorticoids (Cortisol);
- drugs that reduce uric acid levels (Allopurinol).
When you need to determine ferritin, you need to take the test in the morning (often laboratories accept biomaterial for research before 12 noon).
Venous blood is collected in the following sequence:
- A tourniquet is applied to the middle part of the shoulder.
- The patient clenches and unclenches his fist several times to fill the cubital vein with blood.
- The elbow bend is treated with medical alcohol.
- The ulnar vein is punctured with a syringe or a vacuum system, and the tourniquet is removed.
- After obtaining a sufficient amount of blood, the needle is removed and a swab soaked in alcohol is applied.
- The patient is required to hold his arm in a bent position to form a blood clot at the site of the puncture of the vessel.
Iron promotes the formation of hydroxyl radicals through the reaction of hydrogen peroxide with iron ions, which subsequently damages DNA and RNA molecules, as measured by the 8-oxoGuo marker in urine.
The 8-oxoGuo marker is increased in urine in patients with genetically determined high blood iron, as determined by Mendelian randomization.
Mendelian randomization is a method used in epidemiology when causation is inferred from associations between genetic variations that modify a modifiable risk factor and a disease.
Also, the relationship between increased ferritin (iron) in the blood and increased levels of RNA damage by the 8-oxoGuo marker in urine was shown in mice whose tissues were exposed to ferrous sulfate.
Studies in mice have shown that a marker of RNA damage increases monotonically with increasing levels of ferritin in the blood. And this could be prevented by iron chelation therapy.
- www.ncbi.nlm.nih.gov/pubmed/29157668
The ferritin molecule consists of a crystalline core (iron phosphate and hydroxide) and a hollow protein shell (apoferritin). Its synthesis is carried out by cells of the liver, kidneys, bone marrow, spleen, and small intestine.
1/5 of ferritin consists of iron - it is found in it in a physiologically accessible, non-toxic, water-soluble form. The amount of this trace element can reach 3000 atoms. Based on their origin, ferritin is divided into two types:
- tissue - enters the blood when tissue cells containing this protein are destroyed;
- plasma - enters the blood when plasma cells are destroyed.
Since ferritin is a depot of iron in case of unforeseen circumstances, an analysis for this protein accurately shows the amount of reserves of this microelement.
The exact amount is determined by blood serum. For this reason, ferritin is called serum ferritin. The results of the analysis show its volume in micrograms (mcg) or nanograms (ng) per 1 liter of blood.
- It is an acute phase protein, which is necessary to provide nonspecific protection of the body before the development of an immune reaction. This condition is accompanied by fever (fever and chills), which reduces the resistance of pathogenic microorganisms to external factors.
- Used for the synthesis of enzymes - proteins that accelerate chemical reactions.
- Promotes the transformation of the toxic divalent form of iron into a harmless non-toxic one.
- Controls the attachment of iron atoms to transferrin and its transport from mother to fetus.
Functions and role of the element
Without normal amounts of transferrin (Tf), the body does not receive enough iron. This hits hard on all systems. Immunity decreases, the blood cannot carry oxygen due to lack of hemoglobin. This is only a small part of the problems.
The functions of the connection are not so obvious. There are many nuances that are not reflected in the name of the substance.
- The main task of transferrin is to transport iron and provide it to tissues. Indeed, this is a key function. The element enters the body in the form of ions. Positively charged particles.
They have a valency of three. That is, they can attach 3 atoms of another substance to themselves. Designated as Fe3+. In this form, free iron is dangerous for the body. These are toxic ions.
Under the influence of digestive juices and acids, the element transforms into a divalent form. Fe2+. Already in the duodenum, iron is reduced back to Fe3+, which allows it to combine with ferritin and then bind to transferrin.
In this form, the substance moves throughout the body. This is an ongoing process. Any deviations carry risks.
- Oddly enough, the compound is involved in the functioning of the immune system. Although in an indirect way. After the body begins to defend itself, transferrin moves to the site of active inflammation or other damage. It takes iron from tissues and cells.
Without this element, bacteria and other dangerous agents cannot live normally. Thus, the protein creates conditions unsuitable for the functioning of the infection.
- In addition, transferrin plays the role of a cleaner. It utilizes iron that remains after the death of red blood cells. Red blood cells are destroyed constantly and in large quantities. They leave behind iron. Transferrin collects it and uses it again.
This is important for another reason. It was previously said that ferric free iron ions are toxic. But it is in this form that the element remains after the death of red blood cells. Protein solves two problems at once.
- Transferrin is involved in providing reticulocytes, young red blood cells, with iron. He knows how to recognize these cells.
- Indirectly, substance indicators are used in diagnostics. The fact is that the concentration of the compound drops as soon as the inflammatory process begins. Moreover, there is a direct proportion: the more intense the damage, the lower the level of the substance. This is a negative, but informative phenomenon. Especially if doctors conduct in-depth diagnostics.
Transferrin also has a negative feature. With radiation damage, it intensifies radiation sickness. The fact is that siderophilin (another name for the compound) is capable of binding not only iron, but also plutonium ions.
Then it carries dangerous particles into muscle tissue, bones, and organs. This is guaranteed to increase the manifestations of radiation sickness and its severity.
Thus, the role of transferrin in the body is predominantly transport, and indirectly the substance is involved in the functioning of the immune system, hematopoiesis, and some metabolic processes.
How does low ferritin manifest?
Ferritin is our iron “depot”.
The simplest and most banal thing is difficulty getting up in the morning, weakness, lethargy, hair loss, pale skin, bruises under the eyes. Anemia is also indicated by low hemoglobin and an insufficient number of red blood cells. There is also a high risk of anemia in pregnant women - iron reserves are greatly depleted at this time. But this is “oxygen” for the fetus. Therefore, it is especially important for pregnant women to monitor ferritin. If you are just getting ready to become a mother, then start increasing the level in 4-6 months.
The main reason is poor absorption of iron by the walls of the small intestine.
Other reasons:
- Increased estrogen, progesterone - accelerates metabolism (iron metabolism).
- Excess calcium, zinc – slows down the absorption of Fe.
- Major blood loss.
- Abuse of caffeinated drinks.
- Taking antibiotics and hormonal drugs that accelerate general metabolism and reduce blood acidity.
- Iron-deficiency anemia.
- The diet contains few foods containing Fe.
- Damage to the gastrointestinal mucosa.
- Dysbacteriosis 2-3 degrees.
- Long-term use of contraceptives.
- Starvation.
Causes of elevated ferritin:
- Acute diseases accompanied by inflammation.
- Decreased levels of female sex hormones.
- Calcium deficiency; vitamins A, C.
- Extensive burns.
- Alcohol intoxication of the liver and brain.
- The onset of any autoimmune disease.
- Blood transfusion.
- The period of engraftment of a transplanted organ or its rejection by the immune system.
- The course of complex viral diseases: HIV, hepatitis.
- Long-term use of antifungal drugs.
- Excess vitamin K.
- Long-term intake of strong antioxidants – high ferritin and low hemoglobin.
- Leukemia.
- Hemolytic anemia.
- Malignant formations.
In infectious and immune diseases, including during anemia, serum ferritin increases only in the initial stages of the disease. During prolonged periods, its content decreases in the blood serum. But high concentrations can be observed in tissues. High ferritin indicates the ability of the immune system to fight the disease.
Ferritin
Ferritin is a protein that can bind large amounts of iron and is therefore the main form of iron storage in the body. The most ferritin is found in the liver, spleen and bone marrow, since these are the organs that consume iron to build other substances. Normally, a small part of ferritin circulates in the blood, and this amount is proportional to its total content in the body. Therefore, ferritin reflects iron stores in the body.
The level of ferritin in the blood decreases with iron deficiency, so determining the level of this protein is a marker of iron deficiency even before the development of anemia.
In addition, ferritin is an acute phase protein, so its concentration in the blood increases not only with excess iron in the body, but also during inflammatory processes.
How to increase, how to treat?
The reader may have noticed that ferritin testing is often used for differential diagnosis of various pathological conditions, which, of course, entails a differential approach to their treatment. Answering the question of how to increase ferritin, I would like to go back and remember what kind of substance this is and what its biological significance is, and, based on this, decide what measures should be taken if ferritin levels are low. You can increase it in the same way as increasing the iron content in the blood, that is, by consuming it with food. However, will there be any point in promoting yourself if you don’t know:
- What is the fate of the element (Fe) entering the body?
- Can it be safely absorbed in the intestines and delivered to tissues?
- Are there other disorders in the body that provoke a drop in ferritin concentration?
Such questions are most likely within the competence of the doctor, who, if necessary, prescribes a drug with a similar name - Ferritin. Or the iron-containing drug “Cosmofer”, intended for intravenous and intramuscular administration.
IDA treatment regimen
These drugs are used under laboratory control, because, unlike iron, which comes from food and cannot lead to an excess of the element in the body, synthetic ferritin in capsules or in solution can accumulate and create an excess supply, which can lead to side effects. Without proper laboratory monitoring at home and not having sufficient knowledge regarding all the movements of iron in the body, it is better not to try to do it on your own and not to turn iron-containing preparations into a medicine intended for the home medicine cabinet, like analgesics, antispasmodics, antipyretics.
Iron supplements are not at all cheap, they have a lot of restrictions and contraindications; if used thoughtlessly, they can lead, at best, to overaccumulation of the element in the liver with the development of an inflammatory process, and at worst, cause not only allergic reactions, but also anaphylactic shock. Among other things, the patient, when starting treatment, must be absolutely sure that he has iron deficiency anemia, and not some other form where ferritin may be superfluous. It is unlikely that, having gleaned some information, even from reliable sources, a person who does not fully understand the issue will be able to thoroughly understand everything, so self-medication may turn out to be not only inappropriate, but in some cases harmful. It’s worth thinking about, because everyone should mind their own business...
What is the function of transferrin?
Where and from what is transferrin formed?
By its nature, transferrin is a glycoprotein.
It has a molecular weight of about eighty kilodaltons. When performing electrophoresis of blood serum proteins, transferrin will be located in the zone of beta-fraction globulins.
Transferrin is a protein, that is, the monomers for its synthesis will be amino acids. The formation of this protein occurs in the liver and reticuloendothelial system.
The main functions of transferrin
The structure of transferrin has two regions that can bind to iron. That is, the main function of this glycoprotein is the transfer of this trace element to organs and tissues.
Fe2+ enters the body with food. Next, it is oxidized to Fe3+. So, the carrier protein is capable of binding only the oxidized form of this trace element.
Normally, the microelement binding centers in the transferrin molecule are not completely saturated; the rest remains in reserve. In the case of low Fe content, the amount of transport protein in the body increases and all binding centers are filled.
Interesting Facts
The following are interesting facts about this indicator and more:
- Transferrin is also called siderophilin, which literally stands for iron-loving.
- 1 gram of transferrin is capable of binding 1.4 milligrams of Fe.
- Iron has a positive charge, so to bind it in the siderophilin molecule, a negatively charged bicarbonate is additionally required.
- If the body does not have this microelement (iron), then this protein can also bind Cr2+, Mn3+, Cu2+.
- There is a polymorphism of siderophilin that can be detected by electrophoresis. For example, Caucasians have type C, and Africans have type D.
- There are other indicators that characterize iron metabolism in the body and have some relation to transferrin: the ability of serum to bind iron, the percentage of transferrin saturation, etc.
- Fe plays an important role in our body. This element is involved in the formation of another transport protein molecule - hemoglobin. For those who don’t know, hemoglobin transports gases between different tissues. In addition, iron is part of the myoglobin found in muscles, acting as an oxygen depot there. Proteins such as cytochromes also contain this microelement. Their main function is to provide cells with energy, as well as to neutralize toxic compounds.
- The body should receive 10 - 15 micrograms of Fe per day. In some conditions, the need for this microelement increases, for example, during pregnancy.
- If there is an insufficient amount of amino acids in the body, then the processes of transferrin synthesis will be reduced.
- Free Fe ions can contribute to the formation of radicals, which will lead to damage to cell membranes.
Prevention and treatment
In the simplest cases, when the cause is iron deficiency anemia, the prevention of this condition will be a balanced, proper diet. It is impossible to cure low ferritin directly; you can only eliminate the root cause of its decrease.
This may be a chronic disease that also needs treatment. Pay special attention to this if your illness is characterized by blood loss (ulcers). A standard preventive measure not only for monitoring ferritin, but also for the body as a whole, is periodic general tests.
For pregnant women, doctors can select combination medications that help eliminate iron and folic acid deficiency. This will help not only maintain your health, but also provide your baby with everything necessary for growth. Moderate physical activity also has a positive effect on health.
If it is important for you to normalize your diet, then introduce more apples, pomegranates, and grapes into your diet. Meat must be present, preferably beef or veal. Rice and eggs will also help prevent this problem.
Any medicines containing iron are taken strictly under the supervision of a doctor with constant laboratory monitoring. As you can see, ferritin is important for the human body, since it takes part in a number of vital processes. That is why such close attention must be paid to the prevention of iron deficiency.
When is it necessary to determine serum ferritin levels?
A ferritin test is ordered along with other iron tests - when a complete blood count shows that a person has a low hemoglobin level and hematocrit, red blood cells are smaller and less saturated with hemoglobin than usual, i.e. suggest iron deficiency anemia even if other clinical symptoms are not clearly evident.
In the early stages of iron deficiency , no physical effects are usually visible. If a person is healthy, symptoms rarely appear before the hemoglobin level in the blood drops below 100 g per liter. However, as iron deficiency progresses, symptoms eventually begin to appear and your doctor may order ferritin as well as other iron-related tests. The most common symptoms of iron deficiency anemia include:
- chronic fatigue,
- weakness,
- dizziness,
- headache,
- pallor.
As iron stores continue to deplete, shortness of breath, tinnitus (ringing in the ears), drowsiness, and irritability may occur. As the severity of anemia progresses, chest pain, headaches, leg pain, shock, and even heart failure may occur. In addition to the general symptoms of anemia, there are certain symptoms that are specific to iron deficiency anemia: these include cravings for specific substances such as licorice, chalk, dirt or clay, a burning sensation in the tongue or a smooth tongue, sores in the corners of the mouth, toes and nails in spoon form.
Norms by gender and age: table
The norms of this protein in the blood can be determined in laboratory conditions, and each age group has its own reference values.
| Age | Norm, µg/l |
| Newborns in the first 30 days of life | 25–200 (no more than 600) |
| Infants aged 1 to 2 months | 200–600 |
| Infants aged 2 to 5 months | 50–200 |
| Children from six months to 12 years | 7–140 |
| Teenage boys and men | 30–310 |
| Teenage girls and women | 22–180 |
| Pregnant women in the first trimester | 56–90 |
| In the second trimester | 25–74 |
| In the third trimester | 10–15 |
As can be judged from the table, the highest level is observed in the body of newborns - this is due to the fact that immediately after birth, the child’s blood circulation and hematopoietic system begins to be rebuilt to work autonomously. In his body, red blood cells are actively breaking down and other important changes occur - after the completion of the processes, ferritin gradually decreases, and, as a rule, stabilizes by the age of twelve. In adult girls and women, due to anatomical features, the concentration of the compound is lower than in men, and during pregnancy it decreases even more, since all useful substances, including hemoglobin, are taken from the mother’s body by the developing fetus.
Preventive measures
Low ferritin in humans can be treated preventatively. Firstly, it is imperative to diversify the menu. Blood counts must be used.
Preventive measures consist of taking a drug that contains iron and folic acid, in addition, the doctor may prescribe some physical exercise. So, you can see for yourself that ferritin can affect various processes. Many have never even thought about this protein, and how many negative consequences it has. Therefore, it is imperative to monitor indicators in pregnant women and children.
When the quantity is reduced
One of the common diseases accompanied by a decrease in ferritin is iron deficiency anemia . A lack of iron leads to the fact that hemoglobin, the most important component of red blood cells that carries oxygen throughout the body, is not produced in sufficient quantities.
Why is the decline happening?
The most common reasons for a decrease in the concentration of this protein in the blood serum:
- Iron-deficiency anemia;
- celiac disease;
- hemolytic anemia and intravascular hemolysis;
- malabsorption syndrome - impaired absorption of microelements in the intestine;
- severe kidney damage (nephrotic syndrome).
What is dangerous if ferritin is below normal? The fact is that in an iron deficiency state, the production of hemoglobin, the main oxygen carrier, decreases . Consequently, all tissues of the body do not receive sufficient nutrition and experience oxygen starvation. This especially affects the brain and cardiovascular system.
Learn more about anemia from the video:
Association of low concentrations with cardiac pathologies
Iron deficiency and, as a result, anemia, can be caused by heart failure. Moreover, in addition to low ferritin, tests show low hemoglobin levels ; When examining red blood cells, it turns out that they are small in size compared to the norm and are less saturated with hemoglobin.
As anemia progresses, low levels of transferrin saturation may be detected . There is also constant low blood pressure.
But most often, a lack of ferritin is not a consequence, but a cause of cardiovascular disease.
Iron deficiency leads to the following disorders in the functioning of the heart and blood vessels:
- carditis;
- vascular damage;
- metabolic disorders in the myocardium;
- tachycardia.
Since the heart does not receive enough nutrition, it works at an unusual pace and wears out quickly . Due to constant load, it expands and hypertrophies. And this leads to the fact that the myocardium needs increased oxygen supply, which the body is not able to provide.
A decreased or increased serum ferritin concentration indicates that a person is suffering from hemochromatosis or anemia . These conditions have a negative impact on the health of the heart and blood vessels and lead to the development of heart failure, heart attacks and strokes.
Everything in the body is interconnected. Every function, every organ is controlled by hormones and enzymes. And ferritin is one of them. Changes in the normal amount of this hormone can trigger the occurrence of iron deficiency anemia. And one of the manifestations of the disease is hair loss. This symptom causes every lady to panic, since thick, beautiful hair has always been identified with well-groomedness and beauty.
What is the essence of the analysis for the determination of siderophilin in the blood?
One of the main, more accessible methods for determining siderophilin in the blood is turbidimetry .
This method requires blood serum. To obtain it, donated by the patient centrifuged For this purpose, venous blood is most often taken.
A reagent containing antibodies to human transferrin to the patient's blood serum If transferrin is present in the serum, antibodies will specifically bind to it . This to become cloudy . Based on this turbidity, the concentration of siderophilin will be calculated.
According to the calculated indicator, which is proportional to the degree of turbidity, the concentration of transferrin in the blood is determined.
The turbidity of the solution with the test serum is compared with a blank sample, in which the absence of siderophilin is known.
What does it mean if the level is elevated?
Iron is a toxic and dangerous substance for the body that is not excreted in biological fluids. Excess of this trace element accumulates in the heart, liver, and joints, damaging them over time.
Diseases in which high levels
The following diseases can cause an increase in serum ferritin
- hereditary diseases associated with impaired iron storage;
- liver diseases (hepatitis, alcoholic cirrhosis, obstructive jaundice, tissue necrosis, hepatoma);
- leukemia (myeloblastic or lymphoblastic);
- lymphogranulomatosis;
- infectious and inflammatory diseases (rheumatoid arthritis, osteomyelitis, pneumonia, urinary tract infections);
- systemic lupus erythematosus;
- cancerous tumors;
- blood diseases (polycythemia, anemia);
- burns;
- hyperthyroidism;
- Legionnaires' disease.
Elevated numbers and cardiovascular disease
Diseases of the cardiovascular system associated with hemochromatosis most often affect men . In women, the risk of developing heart disease as a result of hemochromatosis occurs only during menopause. And this is understandable: excess iron is removed from the female body during menstruation.
If hemochromatosis is not eliminated, this condition can lead to dysfunction of the cardiovascular system : coronary heart disease, arrhythmia, heart failure, heart attack, and even sudden cardiac arrest.
cardiac hemochromatosis
can develop , a disease in which the heart muscle acquires a characteristic rusty-brown color, thickens and increases in size.
In this case, cardiosclerosis occurs - the proliferation of fibrous tissue. Subsequently, the contractile function of the myocardium decreases due to atrophic or dystrophic changes in muscle fibers.
Typically, after detecting an elevated ferritin concentration, the doctor prescribes the following tests:
- to determine the total iron-binding capacity of serum;
- genetic test for hemochromatosis;
- ECG and Holter study of the heart.
If there is a risk of coronary heart disease, the analysis will show an increase in the level of ESR and leukocytes. Other characteristic changes will be noticeable:
- an increase in the amount of serum iron to 54–72 µmol/l;
- decrease in the total iron-binding capacity of serum;
- low transferrin content;
- hyperglycemia;
- dysproteinemia;
- increasing the coefficient of transferrin saturation with iron to 60–90%.
If you have cardiovascular diseases, you need to keep the level of iron in your body under control. Your doctor will prescribe appropriate therapy to maintain ferritin levels between 70 and 80 mcg/L .
Cardiovascular diseases can be both a consequence and a cause of increased iron levels in the body. For example, with circulatory disorders associated with heart attacks and strokes, patients experience a sharp increase in serum ferritin .
For more useful information about hemochromatosis, watch the video:
Reasons for the increase
Elevated ferritin signals more serious diseases than those diagnosed by low ferritin.
An increase in ferritin concentration is no less dangerous for the body than a decrease, and can be triggered by the following factors:
- hereditary disorders of iron metabolism;
- severe liver dysfunction;
- inflammatory processes;
- thyroid disease (hyperthyroidism);
- oncological diseases - for example, breast cancer;
- alcohol abuse.
In addition, excess ferritin can be diagnosed during prolonged fasting and uncontrolled use of certain medications.
Changes in the concentration of ferritin in the blood are an important diagnostic factor in identifying a number of dangerous pathologies. Even minor changes in the indicator can lead to unpleasant consequences for the body, so if you detect a decrease or increase in protein levels in the analysis, you should consult your doctor as soon as possible.
- Author: ellistar
Rate this article:
- 5
- 4
- 3
- 2
- 1
(6 votes, average: 5 out of 5)
Share with your friends!