Release form
Tablets with a thin film coating, round, biconvex. A total of five types of tablets are produced:
- Marked "DD". There are 2 tablets in the package. The shell is yellow, the core is white.
- Marked "DJ". There are 5 tablets in a package. The shell is pink, the core is white.
- Marked "DH". There are 17 tablets in a package. The shell is pale yellow, the core is white.
- Marked "DN". There are 2 tablets in the package. The shell is red, the core is white.
- Marked “DT”. There are 2 tablets in the package. The shell is white, the core is white.
Each package of Qlaira contains four types of active tablets, each type containing several different doses of hormones. Two dark yellow tablets contain 3 mg estradiol , five medium red tablets contain 2 mg estradiol and 2 mg dienogest , 17 light yellow tablets contain 2 mg estradiol and 3 mg dienogest , and two dark red tablets contain 1 mg estradiol . The package also contains two inactive white tablets.
https://youtu.be/JHunbnvSkWw
pharmachologic effect
Qlaira is a low-dose combined contraceptive intended for oral use. The drug contains both estrogenic and gestagenic components, due to which the tablets are well tolerated by patients in 99% of cases. In addition to the active tablets, the package contains 2 more inactive tablets, which makes it possible to continuously take the drug. The contraceptive effect is achieved by suppressing ovulation , increasing the thickness of cervical mucus , and also reducing the level of sensitivity of the uterine endometrium to the blastocyst (the initial stage of embryo development).
In addition to the main effect, this drug stimulates a decrease in the intensity and duration of the menstrual cycle, relieves PMS symptoms and minimizes pain symptoms.
Pharmacodynamics and Pharmacokinetics
The estrogen in Qlaira is estradiol valerate , an ester of human 17β-estradiol (1 mg estradiol valerate corresponds to 0.76 mg 17β-estradiol ). This estrogen is different from ethinyl estradiol or its precursor mestranol , used in other COCs and containing an ethyl group 17a, which causes more stable metabolic activity , but at the same time has effects on the liver.
Dienogest (DNG) is a progestogen with previous clinical trials. It has a pronounced effect on the endometrium. Structurally derived from nortestosterone, it has the characteristics of nortestosterone and progestogen derivatives. DNG does not have androgenic activity , but does have antiandrogenic activity.
Pharmacokinetics
Estradiol valerate
Absorption: Following oral administration of estradiol valerate, it is completely absorbed by the intestinal mucosa. After initial absorption, E2V is hydrolyzed to produce E2 and valeric acid through a metabolic process in the gastrointestinal mucosa and liver.
Metabolism: metabolism occurs in the gastrointestinal mucosa and liver. Estradiol valerate undergoes extensive changes in first-pass metabolism, resulting in increased serum . Approximately 95% of an oral dose of estradiol valerate is metabolized before it enters the systemic circulation , so the bioavailability of E2V is approximately 3-5%. Metabolism of E2 in the intestinal mucosa and liver leads to the conversion to estrone (E1) and estriol (E3). Both E1 and E3 are metabolized into sulfate and glucuronide forms . These forms of limited cell penetration partly explain their low effectiveness. Estrone (E1) is converted to estrone sulfate (E1-S) by estrogen sulfotransefare and both can be converted back to E2. Since most of the orally absorbed E2 is converted to E1 and E1-S, E2 levels stabilize. Estrogens are further oxidized by liver cytochrome P450 , affecting the CYP3A4 and 1A2 pathways until completely eliminated.
Distribution: 5% of an oral dose of E2V reaches systemic circulation. E2 is bound to 38% of obligatory sex hormone globulin (SHBG), 60% to albumin , and 3% circulates in free form. The approximate volume of distribution is ~1.2 L/kg. Serum concentrations of E2 remain stable over the 28-day treatment period in the range of 0.0336-0.0647 ng/mL due to the steady state between E2-E1-E1-S. Recycling includes E1-S and estrone glucuronide . The half-life of estradiol is 1.5 hours, the terminal half-life of estradiol after oral administration is 13-20 hours and is dependent on the recirculation of estrogen sulfate .
Excretion: Estradiol and its metabolites are primarily excreted in the urine. 10% is excreted in feces.
Dienogest
Absorption: DNG is almost completely absorbed after oral administration and has high bioavailability (91%). There is no clinically established effect on the rate or extent of absorption of DNG by concomitant meals.
Distribution: DNG has a distribution volume of 46L. It circulates in serum with serum albumin (90%) and 9% in the form of free and bioavailable elements. Serum concentrations of DNG at steady state are: min 11.8ng/ml; max 82.9ng/ml.
Metabolism: The half-life from blood plasma is 12 hours. The substance is almost completely metabolized by cytochrome P450, the CYP3A4 isoform into pharmacologically inactive metabolites.
Excretion: DNG and its metabolites are excreted primarily by the kidneys. 86% of the hormone and its metabolites are eliminated after 6 days.
Qlaira for endometriosis
The basis for the use of Qlaira for the treatment of endometriosis is the presence in its composition of dienogest - progesterone, which has good effectiveness in suppressing foci of pathological growth of the endometrium. However, along with dienogest, Qlaira contains the estrogen hormone, which, on the contrary, will lead to an increase in endometriosis. Therefore, it is better to opt for a specialized drug intended for the treatment of endometriosis - Visanne.
A number of women and gynecologists have used Qlaira to treat endometriosis with positive results. However, such empirical experience is not a basis for trying to make Klaira a panacea for the disease. If endometriosis is not advanced, then Qlaira can be used to treat this pathology. However, if there is no effect from Qlaira in the treatment of endometriosis within 6 months, it is necessary to use other methods of therapy. Read more about endometriosis
Contraindications
The contraceptive drug Qlaira should not be taken:
- Women who are breastfeeding (combined birth control pills should not be taken until the baby is weaned or for six months after birth);
- women with venous thromboembolism , such as deep vein thrombosis or blood clots in the lungs ( pulmonary embolism );
- women with blood diseases, since the substances included in the drug, as well as their effect on the body, increase the risk of blood clots in the veins;
- women undergoing sclerosing treatment for varicose veins ;
- women with two or more risk factors for a blood clot in a vein, such as a family history of deep vein thrombosis or pulmonary embolism under the age of 45 (parent, sibling);
- women with obesity, smoking, long-term immobility;
- women who have ever had a heart attack, stroke or mini-stroke caused by a blood clot in an artery;
- women with sore throat, heart valve disease or irregular heartbeat - atrial fibrillation ;
- women with unstable blood pressure;
- women who smoke more than 40 cigarettes a day;
- women over 50 years old;
- women with severe diabetes;
- women with diabetes , high blood pressure, high cholesterol, migraines ;
- women with breast cancer ;
- women with abnormal vaginal bleeding , the cause of which is unknown;
- women with excess urea in the blood, resulting in damaged blood cells ( hemolytic uremic syndrome );
- women with active liver disease , such as liver cancer, hepatitis ;
- women with impaired bile secretion, which causes jaundice ;
- women with gallstones ;
- hereditary blood diseases known as porphyrias.
Claira
If any of the diseases/conditions/risk factors listed below currently exist, the potential risks and expected benefits of the drug should be carefully weighed in each individual case and discussed with the woman before she decides to start taking the drug. If any of these conditions or risk factors worsen, intensify, or appear for the first time, a woman should consult her doctor, who may decide whether to discontinue the drug.
Diseases of the cardiovascular system
The results of epidemiological studies indicate a relationship between the use of COCs and an increased incidence of venous and arterial thrombosis and thromboembolism (such as DVT, PE, MI and cerebrovascular events).
The risk of developing venous thromboembolism (VTE) is greatest in the first year of taking such drugs, mainly during the first 3 months. An increased risk is present after initial use of COCs or resumption of use of the same or different COCs (after a dosing interval of 4 weeks or more).
The overall risk of VTE in patients taking low-dose COCs (ethinyl estradiol) is 2-3 times higher than in patients not taking COCs, however, this risk remains lower compared to the risk of VTE during pregnancy and childbirth.
VTE can be fatal (in 1-2% of cases).
VTE, manifested as DVT or PE, can occur with all COCs. It is extremely rare when using COCs that thrombosis of other blood vessels occurs, for example, hepatic, mesenteric, renal, cerebral arteries and veins or retinal vessels. There is no consensus regarding the relationship between the occurrence of these events and the use of COCs.
Arterial thromboembolism can be fatal.
In women with a combination of several risk factors or high severity of one of them (for example, complicated heart valve diseases, uncontrolled arterial hypertension, extensive surgical interventions with prolonged immobilization, etc.), the possibility of their mutual strengthening should be considered. In such cases, the total value of the existing risk factors increases. In this case, taking the drug is contraindicated.
The risk of developing thrombosis (venous and/or arterial) and thromboembolism increases:
- with age;
- in smokers (with an increase in the number of cigarettes smoked or an increase in age, the risk increases, especially in women over 35 years of age);
in the presence of:
- family history (for example, venous or arterial thromboembolism ever in close relatives or parents at a relatively young age). In the case of a hereditary or acquired predisposition, the woman should be examined by an appropriate specialist to decide on the possibility of taking the drug;
— obesity (body mass index more than 30 kg/m2);
- dislipoproteinemia;
- arterial hypertension;
- migraine;
- heart valve diseases;
- atrial fibrillation;
- prolonged immobilization, extensive surgery, any operation on the lower extremities or major trauma. In such situations, it is advisable to stop taking the drug (for elective surgery, at least 4 weeks before it) and not to resume it for 2 weeks after the end of immobilization.
The possible role of varicose veins and superficial thrombophlebitis in the development of VTE remains controversial.
The increased risk of thromboembolism in the postpartum period should be taken into account. Peripheral circulatory disorders may also occur in diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell anemia.
An increase in the frequency and severity of migraines while using the drug (which may precede cerebrovascular events) may be grounds for immediate discontinuation of this drug.
Biochemical factors indicating a hereditary or acquired predisposition to arterial or venous thrombosis include the following: resistance to activated protein C, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipid antibodies, lupus anticoagulant).
When assessing the risk-benefit ratio, it should be taken into account that treatment of the relevant condition may reduce the associated risk of thrombosis. It should also be taken into account that the risk of thrombosis and thromboembolism during pregnancy is higher than when taking low-dose oral contraceptives (ethinyl estradiol).
Tumors
The most significant risk factor associated with the development of cervical cancer is persistent human papillomavirus infection (PVI). There are reports of a slight increase in the risk of developing cervical cancer with long-term use of COCs. The connection with taking COCs has not been proven. The possibility of the relationship of these data with screening for cervical diseases and with characteristics of sexual behavior (less frequent use of barrier methods of contraception) is discussed.
A meta-analysis of 54 epidemiological studies found a small increase in the relative risk (RR = 1.24) of developing breast cancer in women currently taking COCs. The increased risk gradually disappears within 10 years of stopping these drugs. Because breast cancer is rare in women under 40 years of age, the slight increase in breast cancer diagnoses in current or recent COC users is small relative to the overall risk of breast cancer. Its connection with COC use has not been proven. The observed increased risk may also be a consequence of earlier diagnosis of breast cancer in women using COCs. Women who have ever used COCs are diagnosed with earlier stages of breast cancer than women who have never used them.
In rare cases, during the use of COCs, the development of benign, and in extremely rare cases, malignant liver tumors has been observed, which in some cases led to life-threatening intra-abdominal bleeding. If severe pain in the upper abdomen, an increase in liver size, or signs of intra-abdominal bleeding occur in women taking COCs, liver tumors must be excluded in the differential diagnosis.
Other states
Women with hypertriglyceridemia (or a family history of this condition) may have an increased risk of developing pancreatitis while taking COCs.
Although slight increases in blood pressure have been described in many women taking COCs, clinically significant increases have been reported rarely. However, if a persistent, clinically significant increase in blood pressure develops while taking the drug, the drug should be discontinued and treatment of arterial hypertension should be started. Taking the drug, if necessary, can be resumed if normal blood pressure (BP) levels are achieved through antihypertensive therapy.
The following conditions develop or worsen both during pregnancy and when taking COCs, but their relationship with taking COCs has not been proven: jaundice and/or cholestatic pruritus, cholelithiasis, porphyria, systemic lupus erythematosus, hemolytic-uremic syndrome, Sydenham's chorea, herpes of pregnancy hearing loss caused by otosclerosis.
In women with hereditary forms of angioedema, exogenous estrogens may induce or worsen symptoms of angioedema.
Acute or chronic liver dysfunction may require discontinuation of the drug until liver function tests return to normal. Recurrent cholestatic jaundice, which develops for the first time during pregnancy or previous use of sex hormones, requires discontinuation of the drug. Although COCs may have an effect on insulin resistance and glucose tolerance, there is no need to change the therapeutic regimen in diabetic patients using the drug. However, women with diabetes need careful monitoring while taking the drug.
Cases of worsening the course of endogenous depression, epilepsy, Crohn's disease and ulcerative colitis during the use of COCs have also been described.
Because estrogens can cause fluid retention, women with heart or kidney failure need careful monitoring.
Chloasma can sometimes develop, especially in women with a history of chloasma during pregnancy. Women prone to developing chloasma should avoid exposure to the sun or ultraviolet radiation while taking the drug.
Effect on laboratory tests
Taking the drug may affect the results of some laboratory tests, including biochemical parameters of liver, thyroid, adrenal and kidney function, the concentration of transport proteins in plasma, for example, CSG and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. These changes usually remain within laboratory limits.
Medical examinations
Before starting to use the drug, it is necessary to carefully evaluate contraindications to the use of the drug based on the woman’s life history, family history, as well as general medical and gynecological examination. The frequency and nature of these examinations should be based on existing standards of medical practice, taking into account the individual characteristics of each patient, but at least once every six months. As a rule, blood pressure is measured, the condition of the mammary glands, abdominal cavity and pelvic organs is checked, including cervical cytology. It is necessary to explain to women that the drug does not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Reduced efficiency
The effectiveness of the drug may be reduced if tablets with active ingredients are missed, gastrointestinal disorders occur while taking tablets with active ingredients, or during concomitant drug treatment.
Insufficient control of the menstrual cycle
While using the drug, especially in the first months of use, irregular menstrual-like bleeding ("spotting" or "breakthrough" uterine bleeding) may occur. Therefore, evaluation of any irregular menstrual-like bleeding should be carried out only after an adaptation period of approximately 3 menstrual-like cycles.
If irregular menstrual-like bleeding recurs or occurs for the first time after previous regular cycles, the possibility of non-hormonal causes should be considered and a thorough examination should be performed to exclude malignancy or pregnancy. Such measures may include diagnostic curettage.
Some women may not develop menstrual bleeding while taking inactive white tablets. If the drug was taken in accordance with the rules specified in the section 'Method of administration and dosage', pregnancy is unlikely. However, if before the first absent menstrual bleeding, the tablets were taken irregularly, or there are no 2 menstrual bleeding in a row, you should not continue to use the drug until pregnancy is ruled out.
There was no negative effect of the drug on the ability to drive a car or operate machinery, however, patients who experience episodes of dizziness and impaired concentration during the adaptation period (the first 3 months of taking the drug) should be careful.
Side effects of Qlaira
- nausea and vomiting;
- abdominal pain;
- migraines or attacks of severe headaches;
- enlargement of the mammary glands and pain in this area;
- weight changes;
- retention of water in body tissues (fluid retention);
- vaginal thrush (candidiasis);
- change in menstrual bleeding;
- spotting or breakthrough bleeding;
- depression;
- lack of sexual desire;
- unstable blood pressure;
- skin reactions;
- irregular brown patches on the skin, usually on the face ( chloasma );
- liver dysfunction;
- gallstones.
Qlaira tablets, instructions for use (Method and dosage)
In accordance with the instructions for use of Qlaira, the tablets are taken daily at approximately the same time, one piece at a time. You should take the drug every day, no matter how often you have sex. The tablets should be swallowed without chewing and washed down with plenty of liquid. Eating does not affect the action of the active substances.
Each package contains 26 active tablets and 2 inactive white tablets.
Typically, the period of use begins with taking the second dark red or white and does not end until the next package is opened. Some women experience bleeding after taking the first tablet of a new package, which is considered completely natural and in most cases does not require specialist intervention.
The tablets should be taken continuously, even if the bleeding has not stopped. This means you must start a new pack of tablets on the same day of the week as your current one.
Using the drug for the first time
Provided that no contraceptives have been taken previously, you should start taking Qlaira tablets on the first day of the menstrual cycle. Taking the first pill is guaranteed to protect against pregnancy. After the birth of a child or abortion, you should consult your doctor before taking the drug.
Taking Qlaira after using other oral contraceptives is carried out the next day after taking the last active tablet of the previous package.
If you miss a dose
Inactive tablets:
If the white tablets (the last ones in the package) were not taken on time, they can be taken at any convenient time, since they do not contain active ingredients. The only rule is that you need to take them in any case, so as not to get confused about the number of days you take active pills, as this increases the risk of developing an unwanted pregnancy.
Active tablets:
Depending on the day of your cycle when one dose of the active pill is missed, you may need to take additional contraceptive precautions. If the time that has passed since the usual use of Qlaira is less than 12 hours, then protection against pregnancy is not reduced and you can take the pill without using additional contraceptives. If the time is more than 12 hours, then protection against pregnancy may be reduced. Depending on the day of the cycle when one pill was missed, it is necessary to use an additional method of contraception.
In normal cases, the tablets should be taken orally in the order indicated on the package, at the same time every day. Do not chew the tablets and take them with a moderate amount of water.
You should take one tablet per day for 28 days. A new package of tablets is opened after the last inactive tablet from the previous calendar package has been taken.
You should stop taking Qlaira and consult a doctor immediately if at least one of the possible signs of a blood clot occurs. They include:
- unusual cough;
- severe pain or heaviness in the chest;
- shortness of breath;
- unusual, severe or prolonged headache or migraine attack ;
- partial or complete loss of vision ;
- slurred speech;
- sudden changes in hearing, smell, or taste;
- dizziness or fainting;
- weakness or numbness in any part of the body;
- severe abdominal pain;
- severe pain, swelling, or discoloration of the legs.
Missed a pill
Missing a pill is most often associated with simple forgetfulness. However, if after taking the tablet for 3–4 hours there was
, then the pill is equivalent to a missed pill, and you should use the rules of conduct in this situation. You will have to take a pill to replace the one you torn out from another package.
If a woman forgot to take a white pill, then she should simply throw it away, since it does not affect contraception in any way. Then continue taking it according to your schedule, counting the day with the white pill not taken as valid.
If any colored tablet is missed, then the procedure is different. When the delay in taking the pill is no more than 12 hours from the prescribed time, you should simply take the pill and then continue to use the drug as usual. Klaira's effectiveness in this situation does not decrease.
If the delay in taking the pill is more than 12 hours, then you need to take the pill as quickly as possible. You can even take two tablets at the same time if one was missed and it’s time to take the next one. After this, take Qlaira as usual. However, delaying taking a pill for more than 12 hours suggests a decrease in the effectiveness of the drug, and the need to use a condom for some time, the duration of which depends on the type of pill missed.
So, how many days should you use a condom after taking the missed pill of each color?1. Dark yellow – no need to use a condom.2. Pink – use a condom for 9 days after taking the missed pill.3. Pale yellow from 8 to 17 in a row - use a condom for 9 days after taking the missed pill.4.
You can take no more than two tablets at a time, so if you miss more than one pill, the chance of pregnancy is high. The closer the missed pills are to white, the higher the risk of getting pregnant when missing pills. If a woman does not menstruate after missing pills, she may be pregnant. In this case, tests should be taken to determine the presence or absence of pregnancy.
Interaction with other drugs
The following drugs may speed up the breakdown of the hormones contained in Qlaira in the liver, making the tablets less effective at preventing pregnancy:
- aprepitants;
- Bosentan;
- barbiturates;
- Carbamazepine;
- Modafinil;
- Nevirapine;
- Oxcarbazepine;
- Phenobarbital;
- Phenytoin;
- Primidone;
- Rifampicin;
- Rifabutin;
- Ritonavir;
- Telaprevir;
- Topiramate;
- herbal remedies containing St. John's wort.
If you regularly take one of the listed drugs, Qlaira is not suitable as a method of contraception, since the contraceptive effect of these pills will be minimal and will not provide the proper level of protection against unplanned pregnancy.
If a patient is prescribed Rifampicin or Rifabutin , it is always recommended to use an alternative method of contraception, since these two antibiotics make birth control pills ineffective. It is also recommended to use an alternative method of contraception when treating with other antibiotics (for example, Amoxicillin, Erythromycin, Doxycycline ).
Antifungals such as Griseofulvin may make the tablets less effective. When taking such drugs, you must use an additional method of contraception throughout the course of treatment and for one month after its completion.
Weight loss drugs ( Xenical, Turboslim and others) when taken simultaneously with Qlaira cause severe diarrhea .
diabetes medications . If you have diabetes, the use of any oral contraceptives should be accompanied by regular measurement of blood sugar levels.
Taking Qlaira and medications to reduce high blood pressure provokes a decrease in the effect of the latter.
Qlaira prevents the excretion of fluid with loss of the effects of diuretics.
The tablets may increase the blood levels of the following drugs, which may increase the risk of side effects:
- Cyclosporine
- Melatonin
- Ropinirole
- Selegilin
- Tacrolimus
- Theophylline
- Tizanidine
- Voriconazole
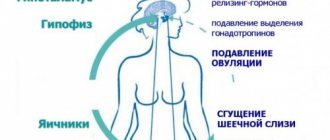
The effect of Qlaira on a woman’s body
The effects of Qlaira are due to two hormones -
. These hormones cause
is suppressed, the egg does not mature, as a result of which pregnancy cannot occur. In addition to the contraceptive effect, Qlaira allows you to reduce the amount of lost menstrual blood by 70% of the initial level, shorten the duration of menstrual bleeding and reduce its pain. Since the amount of blood lost is significantly reduced, Qlaira prevents the development of iron deficiency
among women. The influence of Qlaira on the risk of developing
and endometrium, which this drug reduces.
As an estrogenic hormone, Qlaira contains estradiol valerate, which is a natural component. This is precisely the main difference between Qlaira and other oral contraceptives - the presence of natural estrogen. After all, other contraceptives use synthetic estrogen – ethinyl estradiol.
Estradiol valerate, unlike ethinyl estradiol, loads the liver significantly less and also changes blood clotting rates much less. It also increases the concentration of anti-atherogenic fractions of cholesterol (HDL - high-density lipoproteins) while simultaneously reducing the content of total cholesterol and its atherogenic fractions (LDL - low-density lipoproteins).
The second hormone in Qlaira, dienogest, is a progestogen that provides an antiandrogenic effect, which is subjectively manifested by an improvement in the condition of facial skin, hair and nails, and the removal of acne. Unfortunately, in some women, dienogest can lead to a weakening of sexual desire.
https://www.youtube.com/watch?v=upload
The effectiveness of Qlaira for preventing pregnancy is less than 1 according to the Pearl index. The Pearl index reflects the number of pregnancies occurring per 100 women who used a contraceptive drug for one year. That is, the pregnancy rate with the use of Qlaira is less than 1 in 100 women over the course of a whole year. However, non-compliance with the rules of taking pills or omissions significantly increase the risk of unwanted pregnancy.
Storage conditions
It is necessary to store Qlaira tablets in an airtight container until it is time to open it. If you have already started taking the tablets, they should not be stored longer than the scheduled dosage plan.
Store the drug in a cool, dry place where the temperature does not exceed 30° C.
It is not recommended to store pills or other medications in the bathroom or near the sink. Do not leave it on a windowsill or in a car. Heat and dampness destroy some components of the drug.
The drug should be kept in a place inaccessible to children.
No period
Menstruation on Klayra is most often insignificant, one might say symbolic. Therefore, spotting during the period when your period is due is, in fact, menstruation. This slight discharge should be considered menstruation.
There may be situations where there is no menstruation while taking white pills. In this case, menstruation may occur when taking the first tablets from the second pack, or may not occur at all. If all the pills were taken according to schedule and there were no omissions, then there is no cause for concern - pregnancy in this case is practically excluded. It is necessary to finish the package to the end and monitor the development of menstruation. If the second menstruation does not occur, then you should consult a doctor.
After discontinuation of the drug, menstruation may occur with some delay and be heavy. However, over the course of 2–3 cycles, the body will adjust the functioning of its own ovaries, which were “resting” against the background of Klaira – now it takes time to recover. The delay can be up to 20 – 30 days. In the first month after discontinuation of Qlaira, it is necessary to carefully protect against unwanted pregnancy. This is due to the fact that the body reacts in a unique way to the abolition of combined contraceptives - the risk of becoming pregnant increases significantly.
We suggest you read: Test for alcohol dependence for women - schket
Analogues of Qlaira
Level 4 ATX code matches:
Trisequence
Silhouette
Angelique
Divina
Klaira birth control pills have analogues, including:
- Pharmatex;
- Escapelle;
- Nonoxylol;
- Tri-Regol;
- Noretin;
- Diana-35;
- Gynepristone (used as emergency contraception);
- Nuvaring hormonal ring ;
- Benatex (prescribed as a contraceptive after abortion, childbirth, in the presence of contraindications to the use of other contraceptives, as well as during breastfeeding);
- Erotex (used for local contraception in women of reproductive age);
- Jess;
- Microgynon;
- Lindinet 20;
- Decasol;
- Mirena;
- Logest;
- Yarina;
- Novinet;
- Marvelon;
- Regulon;
- Jeannine;
- Lactinet;
- Charosetta;
- Femoden.
Claira or Janine?
The dosage of hormones in Claira is lower compared to the drug Janine. Therefore, women who are prone to allergies and sensitive by nature are better off taking Qlaira, which has a softer and more gentle effect, not being inferior in effectiveness to Janine. In principle, the choice between Janine and Claira depends on the woman’s age and the existing pathology of the reproductive system.
Janine is more suitable for young girls, and Klaira - for mature women. The drugs differ in the estrogen they contain, and the progesterone component is the same in both. Klaira contains natural estrogen, identical to the natural one, which is produced in a woman’s body by the ovaries, and Zhanine contains a synthetic analogue. Due to this circumstance, preference can be given to Klaira.
Reviews about Klaira
Reviews from doctors about Qlaira indicate the high contraceptive effect that the drug has on women. Experts agree that this drug perfectly protects against unplanned pregnancy and at the same time has a positive effect on the health of the female body as a whole.
Reviews about Qlaira birth control pills are generally varied. Some women claim that taking the drug had an effective effect without the side effects of Qlaira; others experienced side effects, but they were short-lived.
Those who took hormonal pills left reviews about Claira on the forums and most of the reviews were positive or neutral. Women who have used a contraceptive for endometriosis speak especially well of the drug. They claim that specific symptoms disappeared after regularly taking birth control pills. However, the duration of taking the drug is different in all cases.
Interval between taking contraceptives and alcohol
Although Qlaira and alcohol are compatible, you should adhere to certain periods between taking them. So, it is recommended to take a contraceptive 4 hours before or after drinking alcohol. Gynecologists insist on complete abstinence from ethanol, but this is almost impossible for women who take oral contraceptives for several years in a row. Therefore, doctors consider a time interval of 6 to 9 hours between pills and alcohol as an alternative.
https://youtu.be/WKFs7wmE4hE
Experts recommend completely abstaining from alcohol during the first month of taking hormonal contraceptives. This is due to the fact that in the early stages the sensitive female body gets used to the supply of hormones from the outside and adapts to a different mode of operation. This process causes a stressful situation for the female body, which in itself can lead to unexpected consequences. An additional load of alcoholic drinks can increase the side effects of the medicine, weaken or completely eliminate its effect, and also cause other unpredictable consequences. To reduce the danger to your life and health, it is best not to neglect the advice of specialists.
Qlaira price
The price of Qlaira birth control pills is considered affordable for all segments of the population. You can buy this product in pharmacies. The price of Klayra ranges from 800 to 860 rubles per package, which includes 28 tablets.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Qlaira tablets 28 pcs. Bayer Weimar/Bayer Pharma
RUB 1,133 order
Pharmacy Dialogue
- Klaira (tab. p/o No. 28) Bayer
RUB 1,153 order
- Klaira (tab. p/o capt. No. 28 x 3) Bayer
RUB 2905 order
- Klaira (tab. p/o capt. No. 28 x 3)Shering AG
2980 rub. order
Europharm* 4% discount using promo code medside11
- Qlaira 28 tabBayer Pharma AG/Bayer Weimar GmbH
RUB 1,347 order
show more
Pharmacy24
- Qlaira N28 tablets Bayer Weimar GmbH & Co.
KG, Nimechchina 269 UAH. order
PaniPharmacy
- Qlaira tablets Qlaira tablets No. 28 Germany, Bayer Weimar
267 UAH order
show more
Price
Klaira can be purchased at a regular pharmacy or through an online store, with a doctor's prescription. When purchasing the drug, pay attention to the expiration date, which is 4 years from the date of manufacture. If the drug has expired, it should not be used.
The cost of Klaira may vary, as this is due to the costs of transportation, storage, as well as the markup policy of the retail pharmacy chain. Qlaira is produced by only one pharmaceutical company, so there is no difference between the more expensive and cheaper drug. The cost of a package of Klaira in various pharmacies ranges from 650 to 970 rubles.