- Most women experience an improvement in their general condition after hormone replacement therapy.
- pituitary tumor;
- resistant ovarian syndrome;
- organic lesions;
- tuberculosis of the genital organs.
- MRI;
- CT scan of the brain;
- examination of the fundus by an ophthalmologist;
- craniography.
Differential diagnosis
Exhausted ovarian syndrome must be differentiated from the following diseases:
If all of the above symptoms of exhausted ovarian syndrome are present, a differential examination is necessary to exclude a pituitary tumor. For this purpose, the patient is prescribed the following types of diagnostics:
A distinctive feature of hypogonadotropic hypogonadism from wasted bowel syndrome is the presence of follicles in the ovaries. When performing laparoscopy, a decrease in ovarian function and a decrease in their size are detected in women.
Exhausted ovarian syndrome also needs to be differentiated from refractory or resistant ovarian syndromes, since these diseases have many of the same symptoms. For this purpose, morphological examinations of the tissue are carried out.
Often, a woman is also prescribed additional consultation with an endocrinologist, mammologist, psychotherapist and urologist.
Hirudotherapy
Leech treatment is an effective and popular alternative treatment for many gynecological diseases and has been used by healers for centuries.
Hirudotherapy is treatment with medicinal leeches and products derived from them. Hirudotherapy as a treatment method has been known for a very long time, from about 200 BC. This method of therapy was most widely used in medieval and early modern medicine. Currently, leeches are used in gynecology, urology, dermatology, rheumatology and surgery.
Medical leeches are bred in special hirudofarms under sterile conditions and then sold in pharmacies. They are used only once and then disposed of, so the risk of infection is completely eliminated. Medicinal leeches have three rows of jaws with tiny teeth. During the session, live leeches attach to the target area and suck blood. They secrete proteins and peptides that improve the real properties of blood. Leeches leave small, Y-shaped wounds that heal without leaving a scar.
Hirudotherapy for ovarian depletion is indicated primarily because of its complex therapeutic effect on the human body. Leeches for ovarian depletion have a general immunostimulating and thrombolytic effect, improve blood circulation and tissue oxygenation, promote the resorption of inflammatory infiltrates, adhesions, scarring, eliminate stagnation in the pelvic organs and improve lymph flow, normalize hormone levels.
The procedure for applying leeches to a woman’s body does not cause any discomfort and goes quickly. In one session, from two to seven leeches are usually used. Leeches are placed not only vaginally when the ovaries are depleted, but also on different parts of the woman’s body: in the sacrum, lower back, liver, near the anus. As a rule, the therapeutic effect occurs after one or two courses of hirudotherapy for ovarian depletion.
Treatment should only be carried out by an experienced hirudotherapist. Even taking into account all the positive aspects, the use of leeches for the treatment of ovarian wasting syndrome has a number of contraindications. Therefore, you cannot use them for women who are marked by the presence of the following pathological conditions:
- severe hypotension;
- low hemoglobin level;
- blood clotting disorder;
- cancer diseases.
Causes
According to statistics, the frequency of this disease in relation to other diseases reaches 1.6%. Ovarian wasting syndrome is a rather dangerous condition of the female body. However, it is important that before its appearance, women did not experience menstrual irregularities. Many of them have never had problems with reproductive function. Therefore, timely treatment will not only prevent early aging of the female body, but will also make pregnancy possible. In rare cases, patients may need in vitro fertilization to successfully conceive. Such cases are associated with the use of donor biomaterial.
Causes of the syndrome:
- Past streptococcal infection, mumps and rubella.
- Disruption of the hypothalamic regulatory centers in the brain.
- The presence of extragenital pathological processes and gestosis in the mother, which can provoke the appearance of the syndrome.
- Stress overexertion, vitamin deficiencies, poor nutrition or starvation.
- The presence of autoimmune processes in the body, as a result of which antibodies to the ovaries appear.
- Galactosemia.
- Removal of part of the ovaries due to an ectopic pregnancy, myomectomy, or endometrioid cyst. As a result of the operation, the ovarian reserve may be depleted or significantly reduced.
- Significant changes in the follicular apparatus, which can appear under the influence of medications, ionizing radiation, and bad habits of a woman. The supply of cells can be completely exhausted in fifteen years. This development of events will contribute to the onset of early menopause.
- Hereditary factors. Quite often, various chromosomal abnormalities are transmitted from mothers to daughters. In half of the cases, the syndrome appears in women whose mothers experienced early menopause, oligomenorrhea or late menstruation.
Acupuncture
Clinical studies of the effectiveness of acupuncture for early ovarian insufficiency have found that this therapy is an effective treatment for correcting the symptoms of this disease.
In girls diagnosed with ovarian failure, acupuncture used directly over the adnexal area stimulates the normal functioning of the ovaries and increases blood flow to them. Many studies show that acupuncture and Chinese herbs lower FSH hormone levels while increasing estrogen levels. For patients with early ovarian failure, acupuncture has been shown to improve the chances of conceiving.
Fatigued ovarian syndrome is premature ovarian failure caused by a violation of the central regulation of reproductive function in women. Patients under 40 years of age have complaints of:
- cessation of menstruation;
- inability to get pregnant;
- tides;
- sudden mood swings;
- sweating
Essentially, they experience the symptoms of menopause, but at a young age.
Traditional treatment of exhausted ovarian syndrome depends on the causes of the condition, but is usually associated with the prescription of hormone replacement therapy (combined estrogen-progestin drugs). Despite the high efficiency and elimination of unwanted symptoms, long-term treatment with high doses of hormones can lead to tumors of target organs (breasts, adrenal glands). Therefore, some doctors resort to homeopathic remedies for treating this problem.
Prognosis and prevention of pathology
Unfortunately, specific preventive measures to prevent the syndrome have not been developed. The main preventive measures are:
- proper nutrition;
- timely treatment of viral infections;
- avoiding contact with harmful substances;
- elimination of psycho-emotional and physical stress;
- treatment only under the supervision of a physician;
- correct selection of IVF protocol;
- regular preventive examinations.
A woman's diet should be balanced in both calories and nutrients. It is strongly not recommended to use strict diets for the purpose of rapid weight loss. If necessary, a diet should be developed by a doctor.
Viral infections should be treated in a timely manner, carefully following the doctor's recommendations. This applies to both medication and regimen. Contact with hazardous substances at work should be kept to a minimum. It is imperative to use personal protective equipment. To avoid excessive physical activity, you should review your daily routine and occupation. If it is impossible to completely eliminate overload, you need to properly plan your work and rest schedule. It is also advised to reconsider your attitude towards stressful situations. Taking hormonal medications should only be started on the recommendation of a doctor. Before starting therapy, you should undergo a complete laboratory and instrumental examination.
The in vitro fertilization protocol should be selected only taking into account the individual characteristics of the organism. Women should undergo preventive examinations with a gynecologist at least once a year, and patients at risk - once every six months. The Altravita clinic successfully treats women with exhausted ovarian syndrome. If you want to get pregnant and give birth to a child, assisted reproductive technologies using donor eggs are used.
Folliculinum and exhausted ovarian syndrome
Folliculinum is a homeopathic remedy representing the natural follicular hormone of the ovaries. The medicinal effect of the drug is similar to the action of estrogens: together with gestagens, the drug regulates menstrual function and eliminates symptoms of hormonal imbalance.
Folliculinum and exhausted ovarian syndrome are the subject of careful study. It has been proven that long-term therapy with the drug has a positive effect on the endometrium: when taken together with progesterone drugs in a woman’s body, the main monthly hormonal fluctuations are simulated. Thus, the patient will be able to carry and give birth to a healthy child after IVF with a donor egg.
Folliculinum is an oil solution for intramuscular administration in ampoules of 5,000 or 10,000 units (1 ml). The dosage of the drug and the course of treatment should be selected by the doctor depending on the initial level of hormones and the age of the patient.
Homeopathic medicine is contraindicated for:
- oncological diseases;
- mastopathy and other benign formations of the mammary glands; endometritis;
- tendency to uterine bleeding;
- the menopause phase, accompanied by an increased release of female sex hormones.
Treatment of exhausted ovarian syndrome with Folliculinum should be under the supervision of an experienced physician. Criteria for the success of therapy are an increase in the level of estrogen in the blood, improvement in mood, and normalization of menstrual function.
Diagnosis of ovarian failure
Diagnosis of the disease is based on medical history and external manifestations. As a rule, the first menstruation occurs on time, and for the first 10–20 years a woman can independently become pregnant, carry and give birth to a child. Then the level of estrogen decreases, according to the results of functional tests, there is no ovulation. The absence of ovulation is also confirmed by laboratory examination. The level of gonadotropic hormones—follicle-stimulating and luteinizing—increases, and the concentration of prolactin decreases.
During a gynecological examination, the uterus and ovaries are reduced in size. Instrumental methods include ultrasound, laparoscopy and histological examination of ovarian biopsy.
An ultrasound examination reveals:
- reduction in the size of the uterus;
- thinning of the inner lining of the uterus, endometrium.
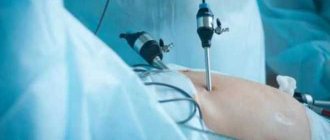
During laparoscopy, changes in the ovaries are detected. The organ becomes small, wrinkled, and acquires a yellowish tint. The corpus luteum and dominant follicle are absent. For a more accurate diagnosis, laparoscopy is supplemented with a biopsy. The resulting material is sent to the pathology laboratory for further study.
The main laboratory diagnostic method is hormone testing. Creating a conditioned cycle with the help of estrogen and progesterone leads to improved well-being. Menstrual-like bleeding begins 3 to 5 days after completing the medication. There is no similar reaction to the introduction of progesterone. This may be due to irreversible changes in the uterine mucosa.
It is noteworthy that the feedback between the ovaries and the hypothalamic-pituitary system is not disrupted. This is confirmed by a decrease in the level of gonadotropic hormones in response to the introduction of estrogens and an even greater increase in response to the introduction of releasing factors. That is, the main diagnostic criteria for exhausted ovaries are:
- Signs of estrogen deficiency during gynecological examination (dry, pale mucous membranes, hypoplasia).
- Ultrasound of the pelvis - decrease in the follicular apparatus of the ovaries, their reduction, absence of signs of ovulation.
- Hormonogram – estrogen deficiency against the background of an increased amount of gonadotropins.
- Hormonal tests with progesterone and combination drugs.
Wasting syndrome should be differentiated from other pathologies that have similar symptoms (resistant ovarian syndrome, hypogonadism, pituitary tumors).
Hormones
One of the main goals of treatment for primary ovarian failure is to replace the estrogen that the ovaries have stopped producing. This is important because the hormones that disappear during ovarian failure are vital to certain processes in the body. Bones, for example, need stimulation from estrogen to remain strong and resistant to fractures.
The main form of estrogen that the ovaries normally produce is called estradiol. For hormone replacement therapy, women can receive it in the form of a pill, skin patch, or vaginal ring. For example, DHEA, when the ovaries are depleted, allows the normal production of testosterone and estradiol in a woman’s body. The estradiol patch and vaginal ring may have a number of advantages over pills:
- They contain the same hormone that the ovaries produce;
- Estrogen does not have to pass through the liver to enter the bloodstream
- Estrogen enters the body gradually, and not immediately in a peak dose.
Despite the benefits that the patch and ring have, other forms of estrogen are also effective.
Most experts now recommend that women diagnosed with ovarian failure take hormones until age 50-51, the average age of menopause.
Symptoms and treatment of Ovarian Wasting Syndrome (OSF). Galactosemia is a possible cause of SIA
obstetrics and gynecology
Ovarian wasting syndrome
Ovarian wasting syndrome (OSF) is presented in the literature under the names “premature menopause”, “premature menopause”, “premature ovarian failure”.
The terms “premature menopause” and “premature menopause” certainly indicate the irreversibility of the process, but their use to characterize a pathological condition in young women is unjustified.
The term “premature ovarian failure” indicates a pathological process in the ovaries, but does not reveal its essence. In addition, an indication of the insufficiency of the function of any organ always implies the possibility of compensation during pathogenetic therapy. In patients with SIJ, therapy aimed at stimulating ovarian function is usually ineffective.
V.P. Smetnik (1980) presents an analysis and critical assessment of the inconsistency of these terms and proposes his own name - “ovarian wasting syndrome.”
The frequency of this syndrome in the population is 1.65%; is one of the forms of premature ovarian failure, the essence of which is that normally formed ovaries cease their function earlier than the usual or expected time of menopause (up to 49.1 years).
The syndrome is manifested by a complex of various pathological symptoms, including amenorrhea, vegetative-vascular changes - “hot flashes”, increased sweating, irritability, decreased ability to work, etc. All these symptoms appear in young women due to premature exhaustion of the ovaries due to disruption of the central mechanisms of regulation of the physiological functions of the female body.
Pathogenesis
- 3 Differential diagnosis.
- 4 Treatment.
Pathogenesis of Ovarian Fatigue Syndrome.
There are a number of theories that explain the causes of ovarian depletion: pre- and postpubertal destruction of ovarian germ cells, chromosomal abnormalities, autoimmune disorders, destructive processes caused by tuberculosis, etc. However, they do not fully reveal the pathogenesis of this syndrome. It is believed that it develops more often in patients with three X chromosome syndrome.
N.V. Svechnikova and V.F. Saenko-Lyubarskaya (1959), M.L. Krymskaya et al. (1965) consider the primary pathogenetic factor of this syndrome to be damage to the central parts of the reproductive system with subsequent involvement of the ovaries in the process. NB Schwarz (1974) shares the same opinion. The author explains the pathogenesis of this syndrome by damage to the ovaries due to increased production of gonadotropic hormones, causing premature atresia of the follicles.
DMSykes and S. Ginsburg (1972), VBManesh (1979) believe that with this syndrome, primary damage to the ovaries occurs. V.I. Bodyazhina (1964), V.P. Smetnik, Z.P. Sokolova (1979) and other researchers, based on the results of studying the functional state and reserve capabilities of the hypothalamic-pituitary system in patients with SLI, agree with this statement . The authors observed stable preservation of the functional state of the hypothalamic-pituitary system and explain the data of their studies by the initial level of gonadotropins in response to the administration of exogenous releasing hormone. Consequently, increased secretion of gonadotropic hormones in these patients occurs secondary to a sharp decrease in the hormonal function of the ovaries.
V.P. Smetnik and E.A. Kirillova (1986) associate the causes of primary ovarian damage with hereditary factors. Based on clinical genetic studies, the authors point to the role of genetic and environmental factors in the occurrence of ovarian wasting syndrome. The genealogical history of patients with SIS in 21.4% of cases turned out to be more genetically burdened (amenorrhea, oligomenorrhea, late menarche, early menopause).
E.A. Kirillova (1989) considers the hereditary cause of this syndrome to be a gene mutation, and the mechanism of inheritance is different in specific families. The author notes that an autosomal dominant type of transmission of the pathological gene is observed, and 10-12% of patients have chromosomal abnormalities in the karyotype.
In 16.4% of cases, patients have menstrual dysfunction; in some cases, similar anomalies were noted in relatives (mother, sister). In addition, the majority of them (81%) had unfavorable factors during fetal development, in the pre- and pubertal periods: gestosis, extragenital pathology in the mother, a high infectious index in childhood.
In addition, the authors also do not exclude the development of this syndrome under the influence of various damaging factors on germ cells in the pre- and postpubertal period, i.e. influence of environmental factors. V.P. Smetnik (1986) admits that against the background of a defective genome, any exogenous influences (infection, intoxication, stress, etc.) can contribute to atresia of the ovarian follicular apparatus.
Galactosemia (with a hereditary disorder of galactose metabolism) cannot be excluded as one of the causes of Ovarian Wasting Syndrome
Consequently, SIS is a multifactorial disease associated with gene diseases, hypothalamic lesions, birth infections, intoxication, stress, starvation, radiation, etc.
V. P. Smetnik (1980) presents detailed data from 52 women examined for the presence of ovarian wasting syndrome. The following methods were used in the examination of these patients: craniography, GHA, PPG, determination of sex chromatin and karyotype, FSH, LH, prolactin, estradiol and cortisol. The history of 65% of women revealed extremely difficult material and living conditions (stress, starvation, etc.), half of them were born during the war. In childhood, there were many infectious diseases: mumps, rubella, chronic tonsillitis - 4 times more often than in the population; during adulthood - intoxication, x-ray exposure, work with toxic substances. 80% of patients had a severe premorbid background. Genealogical data of 28 women was also studied. It turned out that 46.4% of probands had various menstrual dysfunctions. Among 1st and 2nd degree relatives, 13.4% of them had primary infertility. In 21% of patients, the disease began with the appearance of persistent amenorrhea, in the rest - with hypomenstrual syndrome lasting from 0.5 to 5 years with further amenorrhea.
Breast
When examining patients, hypotrophic changes in the mammary glands and external genitalia were revealed; no metabolic and trophic changes were noted. The content of sex chromatin in the nuclei of cells of the oral mucosa is on average (19.3+1.0)%; Karyotype anomalies were found only in 3.5% of cases, which makes it possible to exclude chromosomal aberration as a cause of premature ovarian failure. According to functional diagnostic tests, evidence of severe ovarian hypofunction was obtained: the pupil symptom was negative, basal temperature indicated ovarian hypofunction. The uterine form of amenorrhea was excluded.
When studying hormones, the following was revealed: the level of est-radiol in the blood plasma was (25.8 + 2.3) ng/ml (with a normal range of 40 to 300 ng/ml). Thus, estradiol is practically not synthesized in the gonads of these women. The test with progesterone (gestagens) turned out to be negative. A test with dexamethasone and hCG showed a sharp decrease in cortisol from (53.7 ± 4.1) to (2.2 ± 0.7) ng/ml, which indicates a clear inhibition of the ACTH-adrenal cortex system. During the administration of hCG, no stimulation of the ovaries was detected; the author even notes a decrease in estradiol levels. A test with clomiphene (after 2-3 months) was also negative; no increase in the level of estradiol and CPI was noted. The FSH level was increased 10-15 times, and LH - 4 times. With the introduction of LH-RG, an even greater increase in FSH and LH was noted. After the administration of estradiol, a decrease in FSH is observed. The increase in the level of gonadotropic hormones and their adequate response to the administration of LH-RH allowed us to believe that in SIA, the reserve abilities of the hypothalamic-pituitary system are preserved.
A number of authors express the opinion that autoimmune processes are involved in the genesis of this syndrome. WMHagne et al. (1987) when examining 70 women with secondary amenorrhea at a young age, 4 of them revealed a family tendency to early menopause, 3 out of 50 patients had antibodies to ovarian tissue, and 24 - to other tissues of various organs. MDamewood et al. (1986) with this syndrome in 14 out of 27 patients identified antiovarian antibodies in the cells of the granulosa membrane and in the oocytes in 9 out of 14 patients. When studying cellular immunity, an increase in T cells, especially T helper cells, was revealed, and the number of T suppressor cells and B cells did not exceed those of healthy women. The levels of JgG, JgA and JgM did not exceed those in healthy people. A decrease in the activity of inhibiting the migration of lactophages was also revealed when using AT Haemaphiles influenzae, Candida albicans and vuridase (Mignot MH et al., 1989). Autoimmune phenomena were found in most patients. Taking into account the fact that autoimmune diseases may not manifest clinical symptoms for a long time, the authors believe that further immunological monitoring of women with SIS is necessary. Consequently, they do not exclude the immunological genesis of this syndrome.
Clinic of Ovarian Fatigue Syndrome.
The clinical picture of SIJ most often manifests itself at the age of 37-38 years and develops as a result of switching off the gonad against the background of unchanged function of the hypothalamic-pituitary system with the manifestation of all the symptoms characteristic of estrogen deficiency (Smetnik V.P., 1980). Characteristic is amenorrhea or oligomenorrhea followed by persistent cessation of menstruation. Vegetative symptoms (“hot flashes” to the head) begin after 1-2 months. after the cessation of menstruation, then weakness, headaches, fatigue, pain in the heart, decreased ability to work and other symptoms of autonomic disorders appear. The author believes that menopausal syndrome occurs as a result of switching off the function of the gonads against the background of a kind of diencephalic syndrome and is characterized by numerous symptoms against the background of metabolic disorders. M. Alper et al. (1986) believe that premature menopause in SIS may be cyclical, i.e. Some patients may become pregnant. The authors note that in 6 patients who developed SIA after severe illness, pregnancy occurred after replacement therapy (estrogens, progesterone). Based on this, it has been suggested that exogenous estrogens can sensitize granulosa cells to the effects of FSH and induce ovulation.
The objective status of patients with SUS reveals the following. All of them are of the correct physique, a typical female phenotype. The mammary glands are normal, there is no discharge from the nipples. On gynecological examination, the external genitalia are unremarkable, the cervix and body of the uterus are hypoplastic.
On HSG, the vast majority of patients show a decrease in the size of the uterus and a sharp thinning of its mucous membrane; the fallopian tubes are usually patent.
In PPG, the ovaries are significantly reduced in size, compacted, the external structure is preserved, and the uterus is small.
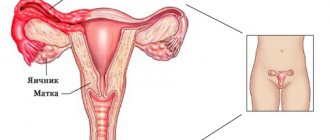
The structure of the internal genital organs of a woman
During ultrasound examination:
- The size of the uterus is small (length 25-30 mm, anteroposterior size reduced to 17-25 mm, transverse - 20-25 mm). The size of the uterus almost corresponds to the II degree of genital infantilism described by M.A. Fuchs et al. (1987). The structure of the uterus is homogeneous, its cavity is visualized in the form of a linear echo signal. The ovaries are reduced in size: length up to 28 mm, width - 17-19 mm, thickness - 19 mm. The structure of the ovaries is homogeneous, moderately hyperechoic, sometimes small, up to 2-3 mm, liquid formations (follicles) can be visualized in the stroma.
During laparoscopy:
- ovaries are reduced in size, yellowish in color. The cortical layer has been transformed into connective tissue, and there is a complete absence of follicles and corpus luteum (Danchenko OV., 1989). The author, during laparoscopy among patients with endocrine infertility, identified SIA in 14.9% of cases. This research method for diagnosing ovarian wasting syndrome is valuable and objective.
Histological examination of ovarian biopsies:
- no follicles are detected, the ovarian stroma is fibrotic in places, with single primordial follicles, or the ovarian stroma with single white and fibrous bodies. Endometrial biopsy - stage of atrophy (Danchenko O.V., 1989). However, with the introduction of estrogen-gestagen drugs, a menstrual-like reaction appears, which indicates the preservation of the sensitivity of endometrial receptors to sex hormones.
Functional diagnostic tests:
- the “pupil” symptom is always negative; the karyopyknotic index is reduced by D° 0-5%, the cervical number is 1-0 points. Basal temperature is monophasic.
Sex chromatin - N; The karyotype was disrupted in only one patient (Smetnik V.P., 1980).
Hormonal status. The FSH level is increased (3 times higher than the ovulatory level and 10-15 times higher than the basal level), on average (118.7 ± 7.4) mU/l; the LH content approaches its level during the ovulatory peak [(51.8+2.3) mU/l]. LҐ/FSH index 0.4:0.2. The secretion of gonadotropic hormones increases secondarily in response to a decrease in ovarian hormonal function. The level of estradiol in plasma is sharply reduced [(28.1+2.4) ng/ml], corresponding to the values after ovariectomy. The amount of prolactin in the blood is slightly reduced.
Electroencephalographic studies by N.M. Tkachenko and V.P. Smetnik (1984) in a number of patients revealed disorders characteristic of the pathology of hypothalamic structures. They manifested themselves as functional changes in the central nervous system, and the authors associate them with the activation of adrenergic structures of the hypothalamus. No irreversible destructive changes were found. After the administration of estrogens, there was a complete restoration of the electrical activity of the brain, which indicated a selective effect of sex steroids on the adrenergic structures of the reticular formation of the brain stem. The authors associate persistent changes characteristic of the electrophysiological activity of the brain with a significant decrease in the level of sex steroids.
Hormonal tests:
- Test with progesterone, no menstrual-like reaction is observed.
- Test with estrogens or gestagens (in a cyclic mode). In all patients, against the background of improvement in general condition, a menstrual-like reaction may appear 3-5 days after progesterone withdrawal, which confirms the severity of ovarian hypofunction and the preservation of the functional activity of the endometrium. These hormonal tests are aimed at identifying the functionality of the gonads and the reactivity of the endometrium.
- Test with dexamethasone and hCG. After the administration of dexamethasone, there is a sharp decrease in the level of cortisol in the blood from (53.7±4.1) to (2.2+0.7) ng/ml, which indicates inhibition of the activity of the ACTH-adrenal cortex system. When hCG is administered, no activation of ovarian function is detected.
- Test with clomiphene. Prescribed 100 mg per day for 5 days. This test is usually negative, i.e. there is no increase in the karyopyknotic index and no increase in basal temperature; the “pupil” phenomenon is negative; the level of estradiol before and after the test does not change.
- Test with estradiol. Aimed at clarifying the pathogenetic mechanisms of impaired secretion of gonadotropic hormones. After the administration of estradiol, a natural decrease in the level of gonadotropins was noted, which indicates the preservation and functioning of the feedback mechanisms between the hypothalamic-pituitary structures and sex steroids (Smetnik V.P., 1986).
- LH-RG test. Positive. It is aimed at identifying the reserve capabilities of the hypothalamic-pituitary system. At the same time, V.P. Smetnik noted an increase in the initially elevated levels of FSH and LH, which indicates the preservation of the reserve capabilities of the hypothalamic-pituitary system.
S.Yu. Kuznetsov (1995) studied the dynamics of some indicators of the blood lipid spectrum and bone tissue density. Significant changes in the lipid spectrum of the blood were revealed in all forms of amenorrhea, including SIJ syndrome, high levels of triglycerides (TG), decreased bone density at points 1/3 and 1/20 of the radius compared with data in healthy women of reproductive age by 9.8 and 25.3%, respectively, which indicates the predominance of bone tissue resection processes in patients with SUS. Based on the results of their studies, the authors explain hypoestrogenism in patients with SI syndrome by metabolic and endocrine disorders, including an increase in the atherogenic potential of the blood. A high content of antiatherogenic lipoproteins in the blood indicates a high risk of developing atherosclerosis and cardiovascular pathology in this syndrome. WJJerber (1994) revealed unidirectional changes in patients with SUS, postovariectomy syndrome and in postmenopausal women.
Osteopenia
Osteopenia in patients after oophorectomy was significantly higher than that in the postmenopausal period. All these changes indicate the risk of developing atherosclerosis, coronary heart disease and osteoporosis. S.Yu. Kuznetsov used treatment for SIJ with anteovin (6 months) and presomin and noted the disappearance of symptoms characteristic of hypoestrogenism. After 3 months After treatment with Presomin, the antiatherogenic potential of the blood was completely restored. The process of demineralization of bone tissue was stopped. Similar results were obtained by WJJerber, S.Polacios et al. (1994). Based on literature data and his own research, S.Yu. Kuznetsov concludes that it is necessary to prescribe hormone replacement therapy to young women with long-term estrogen deficiency in order to prevent the development of atherosclerosis and osteoporosis.
In work carried out at the Department of Endocrinology of the Scientific Center for Aging and Pediatrics of the Russian Academy of Medical Sciences (Smetnik V.P. et al., 2001), bone mineral density (BMD) was established in young women with various forms of amenorrhea and after oophorectomy. The state of BMD of the femur and spine in SSI was similar to that in women after oophorectomy (more than 2-5 years) without the use of HRT.
Summarizing the possibilities of diagnosing SUS, the following methods can be distinguished: a well-collected anamnesis; study of the level of pituitary and ovarian hormones (FSH, LH, estradiol); conducting hormonal tests, ultrasound, laparoscopy and gonadal biopsy. The most valuable for diagnosis are hormonal studies and laparoscopy with ovarian biopsy.
Differential diagnosis.
Ovarian wasting syndrome must be differentiated from resistant ovarian syndrome, pituitary tumor and other diseases.
- Resistant ovarian syndrome is characterized by a complete absence of vasomotor symptoms, moderate estrogen saturation, and occasional independent menstruation. With ultrasound and PPG: the uterus and ovaries are usually of normal size. Macro- and microscopically the ovaries are not changed. The level of gonadotropic hormones is slightly increased. There is moderate estrogen saturation. When large doses of gonadotropins are administered, activation of ovarian function is rarely observed. With this pathology, the follicular apparatus is preserved, cytoplasmic receptors are affected, and therefore menstrual function is disrupted.
- With hypogonadotropic hypogonadism, the level of gonadotropins is low, there are no vasomotor disorders and signs of sexual infantilism. Tests for ovarian stimulation with hCG and clomiphene are positive. During laparoscopy: the ovaries are small, the follicles are visible, their presence is confirmed histologically.
- With a tumor of the pituitary gland, characteristic data are revealed by radiation methods of examination (radiography of the skull, MRI)> ophthalmological, neurological, etc.
- Tuberculosis of the genitals. Characteristic medical history, chronic course of the inflammatory process, infertility. With this pathology, ovarian depletion is possible in a severe process (pyo-ovary).
| diagnostic criteria | Syndrome resistant ovaries | Ovarian wasting syndrome | Gonadal dysgenesis |
| 1 | 2 | 3 | 4 |
| Amenorrhea | Amenorrhea primary or secondary after regular menstrual cycles or rare episodic menstruation | Amenorrhea after a period of regular menstruation and reproductive well-being | Amenorrhea primary or secondary after several irregular menstruation |
| "Tides" | Can be unexpressed "tides" | Severe hot flashes, increased sweating, decreased ability to work. While taking hormonal medications, hot flashes disappear and condition improves | There are no hot flashes or may occur after discontinuation of hormone therapy |
| Vaginal dryness | Not always | Vaginal dryness | Rarely |
| Progesterone test | Positive in 84% of cases | Negative | Negative |
| Test with pergonal | May be positive | Negative | Negative |
| Test for cyclical hormone therapy | Positive | Positive | Positive |
| Phenotype | Female | Female | Underdevelopment of secondary signs: their formation is noted during cyclic hormone therapy |
| Genotype | 46 XX | 46 XX | Mosaicism deletions translocations, monogenic mutations |
| Sex chromatin | Within normal limits | Within normal limits | Reduced |
Treatment.
Taking into account the depletion of the ovarian follicular apparatus, it is inappropriate and not indifferent for the patient to carry out treatment aimed at stimulating ovarian function. Estrogenic hormones, increasing the initially high level of gonadotropins, can contribute to the activation of hyperplastic processes in the target organs for gonadotropins: mammary glands, adrenal medulla (Smetnik V.P., 1980). However, D. Kreiner et al. (1988) proved spontaneous and pharmacologically induced remissions in these patients. In 7 patients with ovarian failure with amenorrhea lasting from 2 to 14 years, ovulation was induced, and in 3 of them pregnancy occurred. Patients received micronized E2 in increasing doses with maintenance doses of progesterone as replacement therapy.
Hormone replacement therapy gives the best results and is etiopathogenetic. Femoston, Klimo-Norm, Klimen, Organametril are used; in young women - Mercilon, Marvelon, Novinet, Regulon, Logest, Silest. At the age of up to 40 years, it is advisable to regulate the cycle differently, then the dose of drugs can be reduced or Femoston, Livial can be prescribed for the treatment of vegetative-vascular disorders, prevention of genitourinary disorders, early atherosclerosis, ischemic heart disease, stroke and osteoporosis. Treatment should be continued until the age of natural menopause.
This therapy should be combined with general somatic and sanatorium-resort methods (physical therapy, acupuncture, massage of the collar area, electrophoresis according to Shcherbak, electroanalgesia, psychotherapy, auto-training; water procedures - circular shower and Charcot shower, iodide-bromine, carbon dioxide, pearl, pine, radon baths) .
Vitamin therapy: vitamins C, E, group B. Sedative therapy: grandaxin, novopassit, valerian, hawthorn, peony.
Non-hormonal drugs containing phytoestrogens include Remens, Klimaktoplan, Klimadinon, Altera Plus.
Products rich in phytoestrogens are sprouted grains of wheat, rye, rice, nuts, berries, soybeans, red clover, Abraham tree, alfalfa, potato juice, sage, ginger, etc.
Rational management of patients leads to normalization of quality of life. Restoration of reproduction is possible when using IVF using donor oocytes.
Ovariamine
In addition to banal hormone replacement therapy, medicine can currently offer a number of alternative drugs to restore and maintain ovarian function in the initial stages of ovarian depletion syndrome.
One such drug that has shown good results in clinical studies in women diagnosed with ovarian failure is ovarian. Doctors assessed the effectiveness of the drug based on a decrease in complaints of menopausal symptoms (hot flashes, mood lability, dyspareunia, etc.) from women, changes in laboratory data of FSH and estradiol levels, and data from instrumental research methods.
Ovariamine is a complex drug obtained by processing bovine ovarian tissue and extracting active components from it. The main active ingredient of the drug is citamines. The medicine is available in the form of tablets for oral administration in dosages of 155 mg and 355 mg.
The nuances of the mechanism of action of Ovariamine on the woman’s body in general and the function of the ovaries in particular still remain unexplored. However, it is known for certain that the components of the dietary supplement help reduce the concentration of follicle-stimulating hormone due to an estrogen-like effect on the female body. A decrease in the blood levels of adrenocorticotropic and thyroid-stimulating hormones was also noted, which further proves the suppressive effect of ovarian on the adenohypophysis. Laboratory indicators and ultrasound data confirm an increase in the volume of the appendages and an improvement in their hormone-producing function.
58% of women who took ovariamin for exhausted ovarian syndrome left reviews indicating that this drug helped improve their monthly cycle and reduce the psychosomatic and psychoemotional manifestations of the disease.
Patients who took ovariamin for ovarian depletion write on the forum about the positive effects of this dietary supplement. They note a decrease and even complete disappearance of symptoms of early ovarian failure such as tearfulness, irritability, sleep disturbances, vaginal dryness and itching, and stress urinary incontinence. More than half of women confirm an improvement in the quality of sexual function and restoration of libido.
Currently, Ovariamine is widely used in patients with various forms of ovarian dysfunction in preparation for an in vitro fertilization protocol, due to the fact that the active substances of the drug help restore the gamete-forming function of the female gonads.
Based on all of the above, we can draw logical conclusions that taking the drug Ovariamin in courses for a year in women with premature ovarian failure syndrome helps to establish metabolism in the tissue of the woman’s gonads, normalize the mechanisms of growth and development of follicles and balance the processes of nervous regulation of the reproductive system. Thus, to such a question as, should I take ovariamin for ovarian depletion or not? Our answer is definitely to drink!
Let's look at how to take ovariamine for ovarian depletion, based on the reviews of those who were helped by ovariamine to get pregnant with ovarian depletion.
Since the drug is a dietary supplement, it is recommended to take an individual approach to prescribing the drug ovariamin for ovarian depletion; the dosage regimen may vary depending on the severity of the symptoms of the disease and the patient’s reproductive plans. The instructions for use of the drug suggest the following regimen: take 1-2 tablets 3-4 times a day 15-20 minutes before meals with a sufficient amount of water. The maximum daily dose of the drug is 1400 mg. In order to achieve maximum effect from the drug, it should be taken in courses of 14-30 days, 3-4 times a year.
We strongly recommend that you pay attention to the drug Ovariamin and use it in the complex treatment of early ovarian failure syndrome.
Traditional medicine
Traditional methods of treatment are included in complex therapy, but do not replace the classical treatment regimen for ovarian dysfunction.
- Exhausted ovarian syndrome is treated with sedatives and infusions. A positive effect on the nervous system was noted from infusions of peppermint (1 tablespoon of dry raw materials per 0.5 liters of water), viburnum, cranberry and blackberry fruit drinks.
- Eliminates psycho-emotional instability freshly squeezed beet juice (100 ml), to which 1 tbsp is added. l. honey The medicine is taken 2 times a day, 50 ml.
- Treatment with folk remedies includes the use of egg-lemon suspension, which effectively combats the manifestations of early menopause due to its high lecithin content. To prepare it, you need to grind lemons and eggshells using a blender in a 1:1 ratio. The resulting mixture is infused for 3-4 weeks, and then consumed 3 times a day, 1 tbsp. spoon.
- You can eliminate the symptoms of ovarian depletion with the help of an infusion of boron uterus. Brew 1 tbsp in 250 ml of water. spoon of dry herb and leave for 30-40 minutes. The infusion is taken up to 5 times a day, 1 tbsp. spoon before eating.
- Infusions of wintergreen, one-sided ortilia, red brush, and field grass, which are prepared in a similar way, have similar properties.
- When the ovaries are depleted, the daily diet must include foods containing lecithin, folic acid, natural phytoestrogens, and B vitamins. These include sprouted grains of wheat, rye, soy, lentils, beans, cauliflower, nuts, fish, egg yolks and others.
- To prepare herbal tea with phytoestrogenic properties, clover, alfalfa, viburnum, sage, ginger, and Abraham tree are used. 0.5 tsp. dry raw materials are brewed in 250 ml of water and left for 15-20 minutes. Take 2-3 times a day for two to three weeks.
Traditional medicine is used only after consultation with the attending physician. Any plant is a potential allergen and, if the body is sensitive, can produce the opposite effect.
Timely identification of the true cause of early depletion of follicular reserves and an integrated approach to treatment prevent loss of fertility and unpleasant symptoms of early menopause.
Read
Also:
- Follicles in the ovaries: normal and abnormalities
- What is an intramural node and how to treat it?
- What is a submucosal node in the uterus and how to treat it?
- Puncture of follicles during IVF, how preparation occurs
Ovarium
Another popular drug on the market for the treatment of ovarian dysfunction is Ovarium compositum. This drug is a homeopathic medicine and has proven itself as an effective medicine in the complex treatment of early ovarian failure syndrome.
Ovarium compositum is a multicomponent medicinal product which includes extracts of the genital organs of farm animals, substances of plant and mineral origin, as well as biocatalysts.
Due to its complex structure and multicomponent nature, the drug Ovarium compositum has a number of effects on the woman’s body, namely:
- normalizes the menstrual cycle;
- accelerates reparative processes in the tissues of the female reproductive system;
- improves blood circulation and lymph flow in the pelvic organs, increasing tissue oxygenation and activating their hidden reserves;
- suppresses tissue alteration during the inflammatory process;
- stabilizes the functioning of the nervous system.
Considering the complexity and wide-ranging action, the use of the drug Ovarium for ovarian depletion is quite common. This is justified by the fact that most doctors who prescribe Ovarium Compositum write reviews that when the ovaries are depleted, this drug as part of complex therapy showed very good results. The well-being of almost all women improved significantly, a regression of menopausal symptoms was observed, and in patients in the initial stages of the disease, in some cases it was possible to restore a regular menstrual cycle.
The natural question is how to take Ovarium Compositum when the ovaries are depleted? The treatment regimen and duration of use in this case will depend on whether the woman menstruates or not.
If a woman is still menstruating, the Ovarium injection regimen for ovarian depletion is adjusted to her menstrual cycle: the first injection is administered the day after the end of menstruation at the time of day when it began. The next four injections are administered at the same time of day at intervals equal to the duration of the last menstruation.
If a woman does not menstruate, then the drug is administered at the same time with a break of 2 days, a total of 5 injections. The course is repeated after a month, for a total of 3-6 courses, or when menopausal symptoms return.
Femoston
The most common drug for hormone replacement therapy on our market is Femoston.
Femoston is a two-component drug that contains estrogen - estradiol hemihydrate and progestin - dydrogesterone. Due to the presence of a progestin component, Femoston is ideal for young women with a preserved uterus, since the gestagen has a protective effect on the endometrium of the uterus and reduces the risk of developing hyperplastic processes.
On pharmacy shelves there are several varieties of the drug Femoston, each of which differs from each other in the dose of the estrogen component. When choosing femoston for ovarian depletion as HRT, a number of points should be taken into account. While during physiological menopause doctors strive to select the minimum effective dose of estrogen in order to minimize the manifestations of side effects, the same dose in women with depleted ovaries will be insufficient, and we will not get the desired results, namely reducing the manifestations of menopausal syndrome, reducing the risk osteoporosis and cardiovascular diseases. In women at the age of physiological menopause, the body's need for estrogen decreases due to the natural aging of the body and the shutdown of a number of its functions. For young women under the age of 45-50 years, the need for estrogen is at a high level, and the hypoestrogenic state due to the failure of the glandular tissue of the ovary is “emergency” and must be replenished in full.
Based on the above, the logical conclusion would be to use the drug Femoston 2/10 for ovarian wasting syndrome, since it contains sufficient estrodiol content that fully compensates for the dose of the hormone normally produced in young women before the onset of physiological menopause.
Ovarian depletion and the use of femoston with it have shown good results in a number of clinical trials. It has been reliably established that taking the drug contributes to the regression of symptoms such as hot flashes, dryness and discomfort in the vagina, dyspareunia, insomnia, and mood lability. Moreover, in women diagnosed with ovarian wasting syndrome and taking femoston, normalization of blood lipid composition was noted, which in turn contributed to the prevention of the development of cardiovascular diseases.
Symptoms of the syndrome
The main sign of pathology is a change in the nature of the menstrual cycle: menstruation becomes irregular with little blood loss, and after a while it stops altogether. Soon after this, the woman begins to notice signs characteristic of menopause:
- Sudden hot flashes, in which there are bouts of heat, when the face and body turns red, while the woman feels heavy sweating, rapid heartbeat, headache and darkening of the eyes, dizziness.
Such attacks may be associated with stress or physical strain, but most often hot flashes occur spontaneously.
- A constant feeling of anxiety, a tendency to depression, increased irritability and other manifestations of psycho-emotional disorder.
- Dryness and itching in the vagina and urethra, resulting in decreased libido and lack of desire for sexual intimacy. This condition is caused by decreased ovarian function and a drop in estrogen levels, which causes the vaginal mucosa to begin to atrophy.
- Women often experience various inflammatory diseases of the genitourinary system, for example, colpitis and urethritis, this is associated with inhibition of the protective properties of the vaginal mucosa.
- Due to a decrease in the production of female sex hormones, a rapid aging process occurs in the body, which primarily affects the condition of the skin.
This entire symptom complex significantly reduces the quality of life of a woman who is still young in age. Hormone replacement therapy helps to cope with such unpleasant factors.
To learn about what exhausted ovarian syndrome is, its causes and symptoms, watch this video:
Claira
In the process of choosing the most successful method of treating early ovarian failure syndrome, it would be a big mistake to forget about the possibility of spontaneous remissions and unpredictable episodes of restoration of a woman’s reproductive function. Let us recall that HRT, which is most often prescribed to patients with SIS, does not have a contraceptive effect.
For women who have not yet managed to realize their reproductive plans, such episodic remissions can be a real blessing. At the same time, for women who have already experienced the joys of motherhood, an unplanned pregnancy can become a serious problem. To avoid such difficult situations, it is worth recommending that patients take oral contraceptives instead of standard HRT. The most optimal drug from this broad category of drugs, which is most suitable for women diagnosed with ovarian depletion syndrome, is Qlaira. The choice of the drug Qlaira for ovarian depletion syndrome is due to the fact that only it contains natural estrogen - estradiol valerate. Taking this COC provides a reliable contraceptive effect and also neutralizes the hypoestrogenic state.
Causes of early, premature ovarian failure
The phrase “early menopause” is often used to denote this pathology, but, according to doctors, it is not correct. Ovarian depletion syndrome resembles menopause only in its symptoms, being essentially a pathology of a different nature. With this disease, there are no signs of premature aging in a woman’s body; it only affects reproductive function.
Structure of the ovary
The etiology of the pathology has not been fully elucidated; gynecologists only talk about the consequences that it causes - the ovaries stop producing follicles and synthesizing female sex hormones.
The number of follicles in a woman is determined at the stage of embryonic development; their formation stops at the time of birth. On average, their number in female ovaries reaches half a million, but only about three hundred thousand mature completely during the entire reproductive period. Follicles mature in girls during puberty, when hormones from the hypothalamic-pituitary system begin to give a signal to the ovaries.
After each menstruation, one follicle in the ovaries becomes smaller; as soon as their reserve is exhausted, the woman experiences menopause. The decline of reproductive function by the age of 45-50 is considered the norm. With ovarian wasting syndrome, the same picture is observed - the supply of follicles has depleted, and the ovaries have lost the ability to maintain reproductive function, but this moment occurs much earlier than the normal age.
We recommend reading about acute salpingoophoritis. You will learn about the causes of acute salpingoophoritis, stages of the disease and symptoms, diagnosis and therapy. And here is more information about the causes and treatment of chronic salpingoophoritis.
Since the etiology of ovarian wasting syndrome does not have an exact scientific explanation, in gynecology it is customary to identify factors that can cause the development of the disease:
- Genetic disorders. Usually, when collecting anamnesis from patients with suspected diagnosis, it turns out that close relatives, for example, a mother or sister, had similar problems. In addition to the early age of cessation of menstruation, hereditary problems with the endocrine system or hormonal balance are also often found.
- Autoimmune pathologies of the endocrine system , for example, dysfunction of the thyroid gland negatively affects the ability of the ovaries to synthesize female sex hormones.
- Neoplasms of various etiologies , various injuries, constant nervous tension, depressive states and other organic and functional disorders significantly negatively affect the functionality of the ovaries. If a woman experiences similar conditions during pregnancy, the process of developing follicles in the female fetus may be disrupted.
- Negative influence of environmental factors : ionizing radiation, toxins, certain medications can cause an inflammatory process in the ovaries, as a result of which there is a risk of replacing functional cells with connective tissue.
- Vitamin deficiencies, especially associated with poor nutrition and diets based on strict restrictions in fats and carbohydrates.
- Surgical interventions during which resection of the ovary was performed in connection with the removal of a tumor or cyst.
If the pathology is based on genetic disorders, this form of ovarian wasting syndrome is called primary, and if the disease develops against the background of healthy ovaries, then it is called secondary.
Other drugs
Committing to a balanced, nutritious diet is an important strategy we have in our arsenal to combat the symptoms and long-term health risks associated with early ovarian failure. In an ideal world, we would get all the nutrients we need from natural sources, but in cases where this is not possible, vitamin and mineral supplements are a vital alternative for women diagnosed with ovarian wasting syndrome. Taking nutritional supplements does not mean you should not eat healthy. But it may help you more effectively relieve the symptoms of premature menopause and combat your increased risk of osteoporosis and heart disease - whether you take HRT or not.
Below are briefly listed vitamins that can help you with ovarian depletion.
Symptoms of ovarian failure
- Menstrual irregularities (first oligomenorrhea, eventually secondary hypergonadotropic amenorrhea).
- Infertility (due to lack of ovulation).
- Symptoms of menopause - sweating, hot flashes, dry mucous membranes, osteoporosis, irritability, sleep disturbances (at first appear periodically and increase over time).
Ovarian wasting syndrome manifests itself quite characteristically. A girl's first menstruation begins during adolescence. Subsequently, against the background of complete health, menstruation becomes less abundant, the intervals between menstruation increase, and secondary amenorrhea develops. Sustained cessation of menstrual function is manifested by symptoms from the autonomic nervous system.
Women complain of hot flashes, sweating, and weakness. Habitual work causes fatigue. Stressful situations cause attacks of anger and aggression, and patients find it difficult to control their emotions. Attacks of headaches and pain in the heart area appear. Sleep is disturbed.
A low amount of estrogen affects the condition of the reproductive system. The mammary glands decrease in size. The glands of the mucous membrane atrophy - atrophic colpitis develops. Bone density decreases - osteoporosis, and the likelihood of fractures increases. There are problems with holding urine.
Get an expert opinion
Leave your e-mail and we will tell you how to get properly examined and begin treatment